Electrochemical mechanism of thioglycolic acid depressing sulphide minerals
来源期刊:中国有色金属学报(英文版)2001年第1期
论文作者:陈建华 冯其明 卢毅屏
文章页码:145 - 149
Key words:TGA; sulphide minerals; electrode potential; separation; pulp concentrate
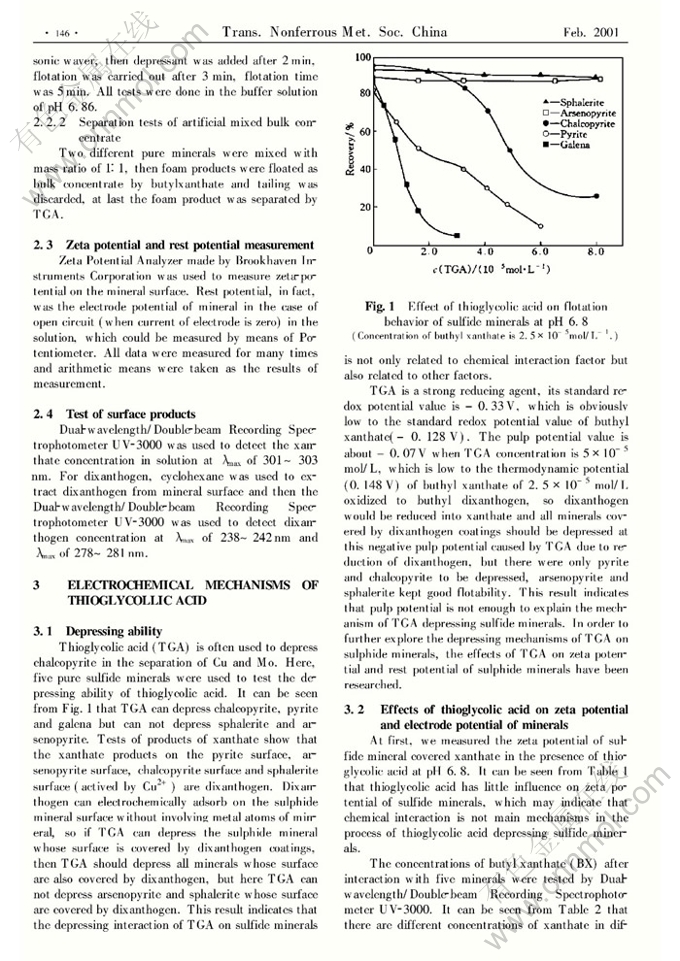
Abstract: The effects of thioglycolic acid (TGA) on the rest potential and zeta potential of sulphide minerals were studied and the electrochemical mechanism of TGA depressing sulphide minerals was put forward. Results of flotation test show that galena, pyrite and chalcopyrite can be well depressed by TGA, but sphalerite and arsenopyrite can not be depressed. Tests also show that TGA has a little influence on zeta potential of sulfide minerals covered by xanthate coatings and TGA can lower the rest potential of sulphide minerals. The electrochemical mechanism of TGA depressing sulphide minerals is that the dixanthogen adsorbing on the mineral surface will be unstable and reduced when rest potential value of sulphide mineral (φMS) is less than the reversible potential of reduction of dixanthogen to xanthate φX-|X2 in the presence of TGA, flotability of sulphide mineral becomes weak; inversely, the coatings of dixanthogen on mineral surface will keep stable when φMS >φX-|X2, sulphide mineral keeps flotability. In the system of mixed minerals, the electrochemical condition of separation of two sulphide minerals by TGA is φMS1 <φX-|X2(φX-|PbX2 ) <φMS2.