Co-invasion of daisy fleabane and Canada goldenrod pose synergistic impacts on soil bacterial richness
来源期刊:中南大学学报(英文版)2020年第6期
论文作者:王从彦 韦梅 王舒 肖鸿光 伍丙德 姜坤
文章页码:1790 - 1801
Key words:co-invasion; Erigeron annuus; invasive plant species; soil bacterial communities; Solidago canadensis
Abstract: Understanding the impacts of co-invasion of multiple invaders on soil bacterial communities is significant in understanding the mechanisms driving successful invasion. This study aimed to determine the response of soil bacterial communities to co-invasion of two invaders daisy fleabane (Erigeron annuus) and Canada goldenrod (Solidago canadensis). Daisy fleabane and/or Canada goldenrod invasion significantly enhanced the operational taxonomic unit richness, Shannon index, and Chao1 index of soil bacterial communities. Canada goldenrod under light degree of invasion and co-invasion of daisy fleabane and Canada goldenrod regardless of invasion degree signally improved the ACE index of soil bacterial communities. Thus, the two invaders can enhance soil bacterial diversity and richness to facilitating subsequent invasion due to the fact that higher soil bacterial diversity and richness can enhance the levels of soil function and nutrients acquisition of plant species. ACE index of soil bacterial communities subjected to co-invasion of daisy fleabane and Canada goldenrod regardless of invasion degree was greater than that under the independent invasion of either daisy fleabane or Canada goldenrod. Hence, co-invasion of the two invaders can impose synergistic impacts on soil bacterial richness, which may build a preferable soil micro-environment via the intensified soil bacterial communities, which is contributive to their following invasion.
Cite this article as: WEI Mei, WANG Shu, XIAO Hong-guang, WU Bing-de, JIANG Kun, WANG Cong-yan. Co-invasion of daisy fleabane and Canada goldenrod pose synergistic impacts on soil bacterial richness [J]. Journal of Central South University, 2020, 27(6): 1790-1801. DOI: https://doi.org/10.1007/s11771-020-4408-9.

J. Cent. South Univ. (2020) 27: 1790-1801
DOI: https://doi.org/10.1007/s11771-020-4408-9
WEI Mei(韦梅), WANG Shu(王舒), XIAO Hong-guang(肖鸿光), WU Bing-de(伍丙德),JIANG Kun(姜坤), WANG Cong-yan(王从彦)
School of the Environment and Safety Engineering, Jiangsu University, Zhenjiang 212013, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: Understanding the impacts of co-invasion of multiple invaders on soil bacterial communities is significant in understanding the mechanisms driving successful invasion. This study aimed to determine the response of soil bacterial communities to co-invasion of two invaders daisy fleabane (Erigeron annuus) and Canada goldenrod (Solidago canadensis). Daisy fleabane and/or Canada goldenrod invasion significantly enhanced the operational taxonomic unit richness, Shannon index, and Chao1 index of soil bacterial communities. Canada goldenrod under light degree of invasion and co-invasion of daisy fleabane and Canada goldenrod regardless of invasion degree signally improved the ACE index of soil bacterial communities. Thus, the two invaders can enhance soil bacterial diversity and richness to facilitating subsequent invasion due to the fact that higher soil bacterial diversity and richness can enhance the levels of soil function and nutrients acquisition of plant species. ACE index of soil bacterial communities subjected to co-invasion of daisy fleabane and Canada goldenrod regardless of invasion degree was greater than that under the independent invasion of either daisy fleabane or Canada goldenrod. Hence, co-invasion of the two invaders can impose synergistic impacts on soil bacterial richness, which may build a preferable soil micro-environment via the intensified soil bacterial communities, which is contributive to their following invasion.
Key words: co-invasion; Erigeron annuus; invasive plant species; soil bacterial communities; Solidago canadensis
Cite this article as: WEI Mei, WANG Shu, XIAO Hong-guang, WU Bing-de, JIANG Kun, WANG Cong-yan. Co-invasion of daisy fleabane and Canada goldenrod pose synergistic impacts on soil bacterial richness [J]. Journal of Central South University, 2020, 27(6): 1790-1801. DOI: https://doi.org/10.1007/s11771-020-4408-9.
1 Introduction
Numerous invasive plant species pose serious risks to the community structure and ecological functions of the invaded habitats [1-6]. Numerous invaders successfully occupy certain environments by altering soil bacterial communities in the rhizosphere and then promote their following invasion [6-12]. Accordingly, the impacts of successful invaders on soil bacterial communities have encouraged ecologists to investigate the successful invasion mechanisms of those invaders.
Through the successful colonization of new habitats by invaders, those species can spread far from their original distribution range [3, 12-14]. Invaders can exhibit different degrees of invasion in the invaded ecosystems [3, 15-18]. Meanwhile, the successful colonization of one invader can construct a micro-environment in the colonized ecosystems that could benefit another invader for succeeding in the previously invaded ecosystems. That is, the colonization of one invader can rise the successful probability of succeeding invaders [1, 2, 15]. Thus, co-invasion of different invaders is common in many invaded habitats [1, 2, 15-18]. Therefore, understanding the impacts of co-invasion of different invaders with different degrees of invasion on soil bacterial communities is significant to fully elucidate the mechanisms driving successful invasion. Previous studies have shown that the sensitivity of soil bacteria may be higher than soil fungi during plant invasion [6, 19]. However, studies on the impacts of invaders on soil bacterial communities mainly focus on single species and/or invasion degree or specific differences between invaders and natives [7, 12, 20, 21]. Existing studies often disregard the invasion degree of invaders or do not focus on differences in soil bacterial communities among various invaders [7, 12, 20, 21].
The purpose of the current study was to assess the impacts of co-invasion of two notorious invaders, daisy fleabane (Erigeron annuus (L.) Pers.) and Canada goldenrod (Solidago canadensis L.), with different invasion degrees, on soil bacterial communities (comparing soil bacterial communities associated with each invader individually, co-occurring invaders, and adjacent uninvaded plant communities) via high-throughput sequencing. The two invaders originate from the Northern parts of North America and are members of the Compositae family, which is the family with the maximal number of species of invaders in China [22, 23]. The two invaders may be found in the same natural habitat [17]. Daisy fleabane is a herbaceous annual species that is distributed across a variety of habitats, for instance, farmlands, forests, and roadsides. Currently, daisy fleabane has been considered one of the most devastating invaders in China due to its multiple ecological effects [15, 17, 24]. Canada goldenrod is an herbaceous perennial species. It was brought to Shanghai, China, as a horticultural species in the nineteen- thirties and quickly spread into most regions of China. Hence, Canada goldenrod has been registered as a notorious and destructive invader in China [3, 15-17, 25].
This study addressed the succeeding hypotheses: 1) soil bacterial diversity and richness rise with growing invasion degree of daisy fleabane and/or Canada goldenrod through the accelerated succession of soil microbial communities mediated by these invaders [7, 21, 26, 27]. This would be consistent with the documented ability of some invaders (e.g., Halogeton glomeratus [26], Acacia dealbata [21], Bromus inermis [7]) for increasing bacterial richness in their rhizosphere. 2) Soil bacterial diversity and richness under co-invasion of daisy fleabane and Canada goldenrod are greater than those under independent invasion of either daisy fleabane or Canada goldenrod.
2 Materials and methods
2.1 Study design
Rhizospheric soil samples of daisy fleabane and/or Canada goldenrod were obtained from three sampling sites (32.207–32.209°N, 119.514– 119.516°E; 32.160–32.161°N, 119.522–119.524°E; and 32.161–32.163°N, 119.528–119.532°E) in Zhenjiang, China, in August 2015. The sampling area has a north subtropical monsoon humid climate. The region has an annual mean temperature of 16.1 °C, a mean annual precipitation of 1150.6 mm, and a mean duration of sunshine 1986.9 h. The climate summaries were obtained from the local records [28].
The plant communities of the sampling site were weed species with daisy fleabane and/or Canada goldenrod as the exclusive invaders. The invasion degrees of the two invaders were divided into light (<35%, L) and heavy (>75%, H) on the basis of their coverage (i.e., canopy coverage) in the same sampling ecosystems [3, 17, 29]. In particular, the light invasion degree mimicked the colonization stage of the invasion process and the heavy invasion degree mimicked the landscape spread stage of the invasion process [13, 14]. Rhizospheric soil samples of daisy fleabane and/or Canada goldenrod were randomly collected from adjacent uninvaded patches, each invader individually at light or heavy invasion degree, and co-invasions with light invasion degree of one and heavy invasion degree of the other invader in three 2 m×2 m quadrats. Three rhizospheric soil cores from each quadrat were obtained through shaking root parts [30], and the cores were homogenized thoroughly to generate soil samples and finally, three soil sample replicates per treatment. The type of collected soil was yellow soil [31, 32].
A total of eight treatment combinations from wildlands were formulated as follows: control (CK, uninvaded), EAL (independent invasion of daisy fleabane with light invasion degree), EAH (independent invasion of daisy fleabane with heavy invasion degree), SCL (independent invasion of Canada goldenrod with light invasion degree), SCH (independent invasion of Canada goldenrod with heavy invasion degree ), EALSCL (co-invasion of daisy fleabane and Canada goldenrod both with light invasion degree), EALSCH (co-invasion of daisy fleabane and Canada goldenrod; the former with light invasion degree and the latter with heavy invasion degree), and EAHSCL (co-invasion of daisy fleabane and Canada goldenrod; the former with heavy invasion degree and the latter with light invasion degree). All replicated soil samples were saved in the closed ziplock bags and cooled at -20 °C in order to better carry out the next disposal process.
2.2 Determination of plant diversity and community composition
Shannon’s diversity index (H'), Pielou’s evenness index (EH), and Simpson’s dominance index (D) were analyzed to assess plant diversity for each quadrat. The results of plant diversity can be referred to as our previous study [15].
2.3 Determination of soil physicochemical properties
Soil pH [8, 9-11] and electrical conductivity [8, 9-11] were tested in situ using the digital soil acidity-moisture meter (ZD-06; ZD Instrument Co., Ltd., Taizhou, China) and the digital soil electrical conductivity tester (ZD-EC; ZD Instrument Co. Ltd., Taizhou, China), respectively. The results of soil physicochemical properties can also be referred to our previous study [15].
2.4 Determination of structure of soil bacterial communities
Soil bacterial communities were identified via high-throughput sequencing (Sangon Biotech Co. Ltd., Shanghai, China). This technique can reveal the changes in soil bacterial communities in response to different invasion degrees of daisy fleabane and/or Canada goldenrod invasion. The amplification of V3–V4 region of bacterial rRNA genes was completed by means of the universal bacterial primers 341F (5′–CCT ACG GGN GGC WGC AG–3′) and 805R (5′–GAC TAC HVG GGT ATC TAA TCC–3′) [33, 34]. The rest of the methods for the determination of soil bacterial communities can be referred to our previous study [12].
2.5 Statistical analysis
Differences in plant diversity, soil physicochemical properties, and alpha diversity of soil bacterial communities among treatment groups were evaluated by analysis of variance (ANOVA) followed by multiple comparisons via Student- Newman-Keuls test. Correlation analysis was achieved using Pearson product-moment correlation coefficient (r) to estimate the relations between plant diversity as well as soil physicochemical properties and alpha diversity of soil bacterial communities. A probability value of P ≤ 0.05 was used to fix statistically significant differences. All statistical analyses were finished through IBM SPSS Statistics (version 22.0, IBM Corp., Armonk, NY, USA).
3 Results
3.1 Alpha diversity of soil bacterial communities
The average Good’s coverage value for the bacterial data across all samples was approximately 77.339% as shown in Figure 1. Data (mean with standard error, n=3) with different lowercase letters indicate a significant difference (P<0.05). Daisy fleabane and/or Canada goldenrod invasion significantly enhanced OTU richness, Shannon index, and Chao1 index of soil bacterial communities compared to control samples (P<0.05). SCL and co-invasion of daisy fleabane and Canada goldenrod regardless of invasion degree also significantly improved ACE index of soil bacterial communities compared to CK (P<0.05). Daisy fleabane and/or Canada goldenrod invasion did not notably impact the sequence number of soil bacterial communities compared to CK (P>0.05). Meanwhile, the ACE index of soil bacterial communities under co-invasion of daisy fleabane and Canada goldenrod regardless of invasion degree was greater than that under the independent invasion of either daisy fleabane or Canada goldenrod, although this change was not dramatical (P=0.0231). Co-invasion of daisy fleabane and Canada goldenrod regardless of invasion degree did not significantly affect sequence number, OTU richness, Shannon index, and Chao1 index of soil bacterial communities compared to independent invasion of either daisy fleabane or Canada goldenrod (P>0.05).
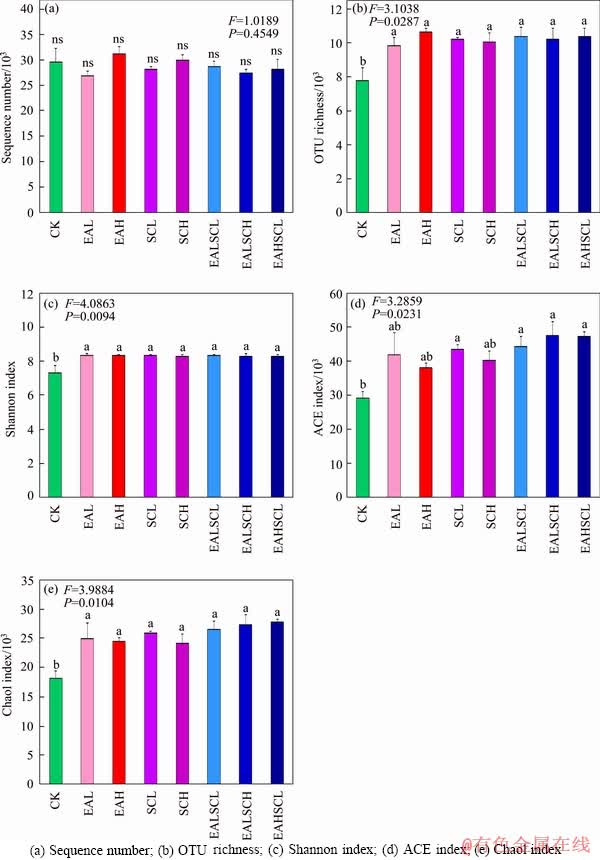
Figure 1 Alpha diversity of soil bacterial communities:
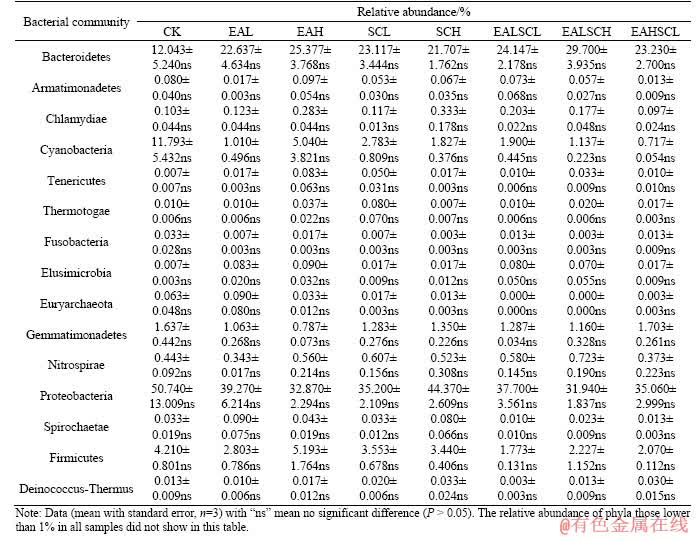
3.2 Relative abundance of soil bacterial community proportions at phylum level
Taxonomic classification of the sequences affords corresponding information on soil bacterial communities and the identity of the detected taxa. In particular, sequences from the Proteobacteria, Bacteroidetes, and Cyanobacteria phyla as well as unclassified bacterial sequences were the most common among all samples (Figure 2 and Table 1). Bars (mean with standard error, n=3) with different lowercase letters indicate a significant difference (P<0.05). The relative abundance of phyla lower than 1% in all samples did not show in Figure 2. At phylum level, relative abundance of Planctomycetes noticeably increased under EALSCH compared to CK (P<0.05). Relative abundance of Chloroflexi dramatically increased under SCL compared to CK (P<0.05). Relative abundance of unclassified sequences notably increased under EAHSCL compared to CK (P<0.05). Relative abundance of Chlorobi prominently increased under EAH degree compared to CK (P<0.05). Relative abundance of Verrucomicrobia distinctively increased under SCH and EALSCH compared to CK (P<0.05). Daisy fleabane and/or Canada goldenrod invasion did not make an obvious shift in relative abundance of the remaining present phyla compared to CK (P>0.05). Relative abundance of Chlorobi under co-invasion of daisy fleabane and Canada goldenrod regardless of invasion degree was markedly lower than that under EAH (P<0.05). Relative abundance of Verrucomicrobia under EALSCH was significantly greater than that under EAL, EAH, or SCL (P<0.05). Co-invasion of daisy fleabane and Canada goldenrod regardless of invasion degree did not signally affect relative abundance of the remaining phyla compared to independent invasion of either daisy fleabane or Canada goldenrod (P>0.05).
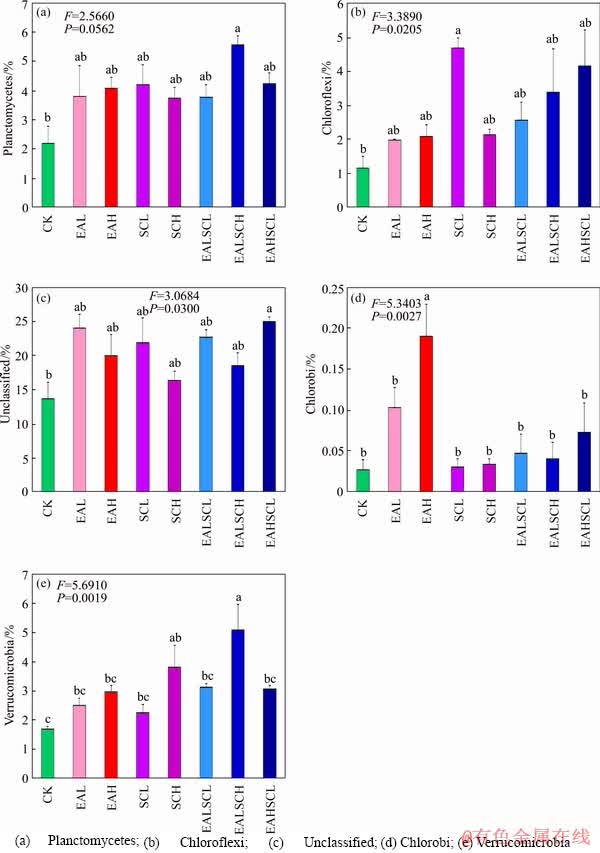
Figure 2 Relative abundance of soil bacterial community proportions at the phylum level:
Table 1 Relative abundance remaining of soil bacterial community proportions at phylum level
3.3 Beta-diversity of soil bacterial communities
Correlation patterns between the beta-diversity of soil bacterial communities were separately surveyed by sample distance heat map paint (Figure 3), which is based on weighted UniFrac distances. Color block represents distance values. Red represents a relatively short distance between the samples as well as a high similarity, and blue color represents a greater distance between the samples as well as a low similarity. Beta diversity of soil bacterial communities under daisy fleabane and/or Canada goldenrod invasion shows obvious differences with CK.
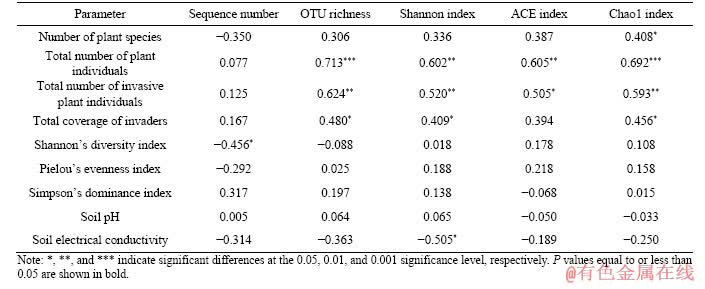
3.4 Correlations between plant diversity as well as soil physicochemical properties and alpha diversity of soil bacterial communities
Correlation analysis was completed to infer the relations between plant diversity as well as soil physicochemical properties and alpha diversity of soil bacterial communities as shown in Table 2. In particular, the number of plant species was positively correlated with the Chao1 index of soil bacterial communities (P<0.05). The total number of plant individuals and the total number of invasive plant individuals were positively correlated with OTU richness, Shannon index, ACE index, and Chao1 index of soil bacterial communities (P<0.01). The total coverage of invaders was positively correlated with OTU richness, Shannon index, and Chao1 index of soil bacterial communities (P<0.05). Shannon’s diversity index of plant communities was negatively correlated with the sequence number of soil bacterial communities (P<0.05). Soil electrical conductivity was negatively correlated with the Shannon index of soil bacterial communities (P<0.05).
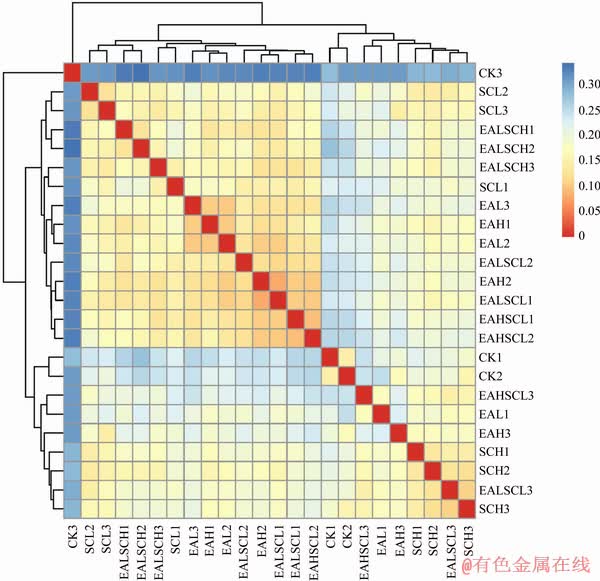
Figure 3 Heatmap of beta diversity estimates of soil bacterial community based on weighted uniFrac distances
Table 2 Correlations between plant diversity as well as soil physicochemical properties and alpha diversity of soil bacterial communities
4 Discussion
Soil pH is widely regarded as a drive index that impacts the abundance and distribution of soil microbial communities [7, 9, 10, 35, 36]. FIERER et al [37] described that soil pH could explain the chief differences in soil bacterial diversity and richness based on ecosystem type. However, no noteworthy relationship between soil pH and alpha diversity of soil bacterial communities was observed in this study. This is consistent with the fact that daisy fleabane and/or Canada goldenrod invasion did not trigger significant impacts on soil pH. Although daisy fleabane and/or Canada goldenrod invasion did not have obvious impacts on soil electrical conductivity, we found a substantial negative relation between soil electrical conductivity and the Shannon index of soil bacterial communities. To a certain extent, soil electrical conductivity can affect soil microbial community structure mainly principally via the altered carbon source utilization strategy of soil microbial communities [8, 9, 11, 15, 36]. This is possibly due to the shifts in ion concentrations arbitrated by root exudates freed from plant species. Previous results also revealed that the concentration of root exudates released from plant species was strongly correlated with soil electrical conductivity [38].
Invaders can change the soil microbial community structure [7, 11, 12, 19, 20, 26] in a way that promotes invaders but restrains natives to accelerate subsequent invasion of the former [27, 38]. Conversely, plant species can also receive feedback from soil microorganisms and then have obvious impacts on the composition and performance of plant communities [7, 11, 12, 19, 20, 26, 39]. This study found that daisy fleabane and/or Canada goldenrod invasion can increase soil bacterial diversity and richness to expedite their subsequent invasion process due to the fact that higher soil bacterial diversity and richness can enhance the levels of soil functions and nutrients acquisition for plant species [39-41]. This result is identified with our hypothesis and previous results [7, 21, 26]. Meanwhile, daisy fleabane and/or Canada goldenrod invasion also increase relative abundance of some phyla based on the taxonomic classification of the sequences. Moreover, total coverage of the invaders was positively correlated with OTU richness, Shannon index, and Chao1 index of soil bacterial communities. Similarly, the total number of invasive plant individuals was also a significant positive correlation with OTU richness, Shannon index, ACE index, and Chao1 index of soil bacterial communities. Hence, total coverage of the invaders and the total number of invasive plant individuals can upsurge soil bacterial diversity and richness. One possible reason for this is that the invaders can release different allelochemicals that have selective impacts on soil microbial communities. In particular, the phenolic acids in root exudates of the invaders (especially Canada goldenrod) stimulated bacterial growth, but the terpenoids in the root exudates of the invaders (especially Canada goldenrod) inhibited fungal growth [26, 42]. Meanwhile, previous results also revealed that two invaders (daisy fleabane and horseweed) significantly increase the bacterial amount and obviously decrease the fungal amount contrarily [43]. Thus, the invaders can generate a preferable soil micro-environment through the strengthening of soil bacterial communities, which can contribute to their next invasion.
In nature, there are diverse invaders in certain invaded ecosystems [1, 2, 15-17]. Variations in plant diversity can directly affect soil microbial community structure mainly via the shifts in the amount, type, and complexity of organic matter input to soil sub-system, especially via litter mixing. In particular, the chemical environment and micro-decomposer communities may be altered when litters are mixed as long as there are species differences in resource quality and structure [44, 45]. Interestingly, these mixture impacts are mostly synergistic owing to the ability of greater plant diversity to support advanced biochemical diversity of leaf litter quality and therefore select for more heterogeneous microbial communities [44, 45]. Consequently, plant diversity can promote soil microbial diversity and functions [15, 46, 47]. Based on this, soil bacterial diversity and richness under the co-invasion of daisy fleabane and Canada goldenrod regardless of the degree of invasion may be greater than those under the independent invasion of either daisy fleabane or Canada goldenrod. The current study showed that the co-invasion of daisy fleabane and Canada goldenrod can increase soil bacterial richness, which is consistent with our hypothesis.
Co-invasion of daisy fleabane and Canada goldenrod regardless of invasion degree also significantly increased the relative abundance of the phylum Verrucomicrobia compared to independent invasion of either daisy fleabane or Canada goldenrod. However, this study also found that co-invasion of daisy fleabane and Canada goldenrod regardless of invasion degree significantly decreased the relative abundance of the phylum Chlorobi compared to the independent invasion of daisy fleabane with heavy invasion degree. This indicated that co-invasion of daisy fleabane and Canada goldenrod may reduce some soil bacterial species while raising soil bacterial richness and relative abundance of some taxonomic groups.
To a certain extent, therefore, co-invasion of two invaders can pose synergistic impacts on soil bacterial richness in their rhizosphere to create a preferable soil micro-environment by the intensified soil bacterial communities, which in return facilitates further invasion. In this study, there are critical differences in plant diversity between the co-invasion of daisy fleabane and Canada goldenrod and independent invasion of either daisy fleabane or Canada goldenrod, especially the total number of plant individuals, number of plant species, and diversity of plant communities. This study showed that the total coverage of the invaders was closely related to OTU richness, Shannon index, and Chao1 index of soil bacterial communities. Moreover, the total number of plant individuals and the total number of invasive plant individuals were strongly correlated with OTU richness, Shannon index, ACE index, and Chao1 index of soil bacterial communities. Lastly, the number of plant species was positively correlated with the Chao1 index of soil bacterial communities, and the diversity of plant communities was negatively correlated with the sequence number of soil bacterial communities. These correlations that soil bacterial communities under co-invasion of daisy fleabane and Canada goldenrod may be primarily impacted by plant diversity via modified resource availability and niche differentiation for bacterial communities in soil.
5 Conclusions
This study determined the impacts of co-invasion at different degrees of invasion of two invaders, daisy fleabane and Canada goldenrod, on soil bacterial communities. The invasion of daisy fleabane and/or Canada goldenrod all exhibited significantly positive impacts on the OTU richness, Shannon index, and Chao1 index of soil bacterial communities. Meanwhile, the independent invasion of Canada goldenrod with a light degree of invasion and the co-invasion of daisy fleabane and Canada goldenrod regardless of the degree of invasion exerted significant positive impacts on the ACE index of soil bacterial communities. Thus, the invasion of daisy fleabane and/or Canada goldenrod can increase the diversity and richness of soil bacterial communities to facilitate further invasion by increasing soil bacterial diversity and enhancing the soil functions and nutrient acquisition of plant species. The co-invasion of two invaders can have synergistic impacts on soil bacterial communities in their rhizosphere to create a better soil microenvironment by improving soil bacterial diversity, which in turn facilitates further invasion. Meanwhile, soil bacterial communities under the co-invasion of daisy fleabane and Canada goldenrod were principally influenced by plant diversity and community composition via changes in resource availability and niche differentiation for bacterial communities in soil.
References
[1] KUEBBING S E, CLASSEN A T, SIMBERLOFF D. Two co-occurring invasive woody shrubs alter soil properties and promote subdominant invasive species [J]. Journal of Applied Ecology, 2014, 51(1): 124-133. DOI: 10.1111/ 1365-2664.12161.
[2] KUEBBING S E, PATTERSON C M, CLASSEN A T, SIMBERLOFF D. Co-occurring nonnative woody shrubs have additive and non-additive soil legacies [J]. Ecological Applications, 2016, 26(6): 1896-1906. DOI: 10.1890/15- 1931.1.
[3] WANG C Y, JIANG K, LIU J, ZHOU J W, WU B D. Moderate and heavy Solidago canadensis L. invasion are associated with decreased taxonomic diversity but increased functional diversity of plant communities in East China [J]. Ecological Engineering, 2018, 112(3): 55-64. DOI: 10.1016/j.ecoleng.2017.12.025.
[4] WANG C Y, LIU J, ZHOU J W, XIAO H G. Differences in leaf functional traits between exotic and native compositae plant species [J]. Journal of Central South University, 2017, 24(10): 2468-2474. DOI: 10.1007/s11771-017-3658-7.
[5] WANG C Y, ZHOU J W, LIU J, XIAO H G, WANG L. Differences in functional traits and reproductive allocations between native and invasive plants [J]. Journal of Central South University, 2018, 25(3): 516-525. DOI: 10.1007/ s11771-018-3756-1.
[6] SOUZA-ALONSO P, GUISANDE-COLLAZO A, GONZALEZ L. Gradualism in Acacia dealbata Link invasion: Impact on soil chemistry and microbial community over a chronological sequence [J]. Soil Biology and Biochemistry, 2015, 80: 315-323. DOI: 10.1016/j.soilbio. 2014.10.022.
[7] PIPER C L, SICILIANO S D, WINSLEY T, LAMB E G. Smooth brome invasion increases rare soil bacterial species prevalence, bacterial species richness and evenness [J]. Journal of Ecology, 2015, 103(2): 386-396. DOI: 10.1111/1365-2745.12356.
[8] WANG C Y, JIANG K, ZHOU J W, WU B D. Solidago canadensis invasion affects soil N-fixing bacterial communities in heterogeneous landscapes in urban ecosystems in East China [J]. Science of the Total Environment, 2018, 631-632: 702-713. DOI: 10.1016/ j.scitotenv.2018.03.061.
[9] WANG C Y, ZHOU J W, LIU J, DU D L. Responses of soil N-fixing bacteria communities to invasive species over a gradient of simulated nitrogen deposition [J]. Ecological Engineering, 2017, 98(1): 32-39. DOI: 10.1016/j.ecoleng. 2016.10.073.
[10] WANG C Y, ZHOU J W, LIU J, JIANG K, DU D L. Responses of soil N-fixing bacteria communities to Amaranthus retroflexus invasion under different forms of N deposition [J]. Agriculture, Ecosystems & Environment, 2017, 247: 329-336. DOI: 10.15244/pjoes/64240.
[11] WANG C Y, WEI M, WANG S, WU B D, DU D L. Cadmium influences the litter decomposition of Solidago canadensis L. and soil N-fixing bacterial communities [J]. Chemosphere, 2020, 246: 125717. DOI: 10.1016/ j.chemosphere.2019.125717.
[12] WANG C Y, JIANG K, ZHOU J W, XIAO H G, WANG L. Responses of soil bacterial communities to Conyza canadensis invasion with different cover classes along a climatic gradient [J]. Clean-Soil, Air, Water, 2018, 46(8): 1800212. DOI: 10.1002/clen.201800212.
[13] THEOHARIDES K A, DUKES J S. Plant invasion across space and time, factors affecting nonindigenous species success during four stages of invasion [J]. New Phytologist, 2007, 176(2): 256-273. DOI: 10.1111/j.1469-8137.2007. 02207.x.
[14] RAI P K. Paradigm of plant invasion: multifaceted review on sustainable management [J]. Environmental Monitoring and Assessment, 2015, 187(12): 759. DOI: 10.1007/s10661-015- 4934-3.
[15] WANG C Y, ZHOU J W, LIU J, JIANG K, XIAO H G, DU D L. Responses of the soil fungal communities to the co-invasion of two invasive species with different cover classes [J]. Plant Biology, 2018, 20(1): 151-159. DOI: 10.1111/plb.12646.
[16] WANG C Y, ZHOU J W, LIU J, WANG L, XIAO H G. Reproductive allocation strategy of two herbaceous invasive plants across different cover classes [J]. Polish Journal of Environmental Studies, 2017, 26(1): 355-364. DOI: 10.15244/pjoes/64240.
[17] WANG C Y, WEI M, WANG S, WU B D, CHENG H Y. Erigeron annuus (L.) Pers. and Solidago canadensis L. antagonistically affect community stability and community invasibility under the co-invasion condition [J]. Science of the Total Environment, 2020, 716: 137128. DOI: 10.1016/ j.scitotenv.2020.137128.
[18] WANG C Y, ZHOU J W, LIU J, XIAO H G, WANG L. Functional traits and reproductive allocation strategy of Conyza canadensis as they vary by invasion degree along a latitude gradient [J]. Polish Journal of Environmental Studies, 2017, 26(3): 1289-1297. DOI: 10.15244/pjoes/66175.
[19] LORENZO P, PEREIRA C S, RODRIGUEZ- ECHEVERRIA S. Differential impact on soil microbes of allelopathic compounds released by the invasive Acacia dealbata link [J]. Soil Biology and Biochemistry, 2013, 57(3): 156-163. DOI: 10.1016/j.soilbio.2012.08.018.
[20] FAN L, CHEN Y, YUAN J G, YANG Z Y. The effect of Lantana camara Linn. invasion on soil chemical and microbiological properties and plant biomass accumulation in southern China [J]. Geoderma, 2010, 154(3, 4): 370-378. DOI: 10.1016/j.geoderma.2009.11.010.
[21] LORENZO P, RODRLGUEZ-ECHEVERRLA S, GONZALEZ L, FREITAS H. Effect of invasive Acacia dealbata Link on soil microorganisms as determined by PCR-DGGE [J]. Applied Soil Ecology, 2010, 44(3): 245-251. DOI: 10.1016/j.apsoil.2010.01.001.
[22] YAN X L, LIU Q R, SHOU H Y, ZENG X F, ZHANG Y, CHEN L, LIU Y, MA H Y, QI S Y, MA J S. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants [J]. Biodiversity Science, 2014, 22(5): 667-676. DOI: 10.3724/SP.J.1003. 2014.14069. (in Chinese)
[23] WANG C Y, LIU J, XIAO H G, ZHOU J W, DU D L. Floristic characteristics of alien invasive seed plant species in China [J]. Anais da Academia Brasileira de Ciências, 2016, 88(3): 1791-1797. DOI: 10.1590/0001-3765201620150687.
[24] WEBER E, GUO S G, LI B. Invasive alien plants in China: diversity and ecological insights [J]. Biological Invasions, 2008, 10(8): 1411-1429. DOI: 10.1007/s10530-008-9216-3.
[25] ZHAO S Y, SUN S G, DAI C, GITURU R W, CHEN J M, WANG Q F. Genetic variation and structure in native and invasive Solidago canadensis populations [J]. Weed Research, 2015, 55(2): 163-172. DOI: 10.1111/wre.12130.
[26] DUDA J J, FREEMAN D C, EMLEN J M, BELNAP J, KITCHEN S G, ZAK J C, SOBEK E, TRACY M, MONTANTE J. Differences in native soil ecology associated with invasion of the exotic annual chenopod, Halogeton glomeratus [J]. Biology and Fertility of Soils, 2003, 38(2): 72-77. DOI: 10.1007/s00374-003-0638-x.
[27] SVENSSON J R, NYLUND G M, CERVIN G, TOTH G B, PAVIA H. Novel chemical weapon of an exotic macroalga inhibits recruitment of native competitors in the invaded range [J]. Journal of Ecology, 2013, 101(1): 140-148. DOI: 10.1111/1365-2745.12028.
[28] HANG Z H, WU H P. Zhenjiang yearbook: Overview of Zhenjiang (The first edition) [M]. Beijing: Publishing House of Local Records, 2017: 30-31. (in Chinese)
[29] WU B D, ZHANG H S, JIANG K, ZHOU J W, WANG C Y. Erigeron canadensis affects the taxonomic and functional diversity of plant communities in two climate zones in the North of China [J]. Ecological Research, 2019, 34(4): 535-547. DOI: 10.1111/1440-1703.12024.
[30] LEI N F, LI J, NI S J, CHEN J S. Effects of clonal integration on microbial community composition and processes in the rhizosphere of the stoloniferous herb Glechoma longituba (Nakai) Kuprian [J]. PLoS One, 2014, 9(9): e108259. DOI: 10.1371/journal.pone.0108259.
[31] ZHANG W L, XU A G, ZHANG R L, JI H J. Review of soil classification and revision of China soil classification system [J]. Scientia Agricultura Sinica, 2014, 47(16): 3214-3230. DOI: 10.3864/j.issn.0578-1752.2014.16.009. (in Chinese)
[32] IUSS Working Group WRB. World reference base for soil resources. International soil classification system for naming soils and creating legends for soil maps [M]. World Soil Resources Reports, 2015.
[33] HERLEMANN D P R, LABRENZ M, JURGENS K, BERTILSSON S, WANIEK J J, ANDERSSON A F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea [J]. ISME Journal, 2011, 5: 1571-1579. DOI: 10.1038/ismej.2011.41.
[34] HUGERTH L W, WEFER H A, LUNDIN S, JAKOBSSON H E, LINDBERG M, RODIN S, ENGSTRANDB L, ANDERSSONA A F. DegePrime, a program for degenerate primer design for broad-taxonomic-range PCR in microbial ecology studies [J]. Applied and Environmental Microbiology, 2014, 80: 5116-5123. DOI: 10.1128/AEM. 01403-14.
[35] KUEBBING S E, SOUZA L, SANDERS N J. Effects of co-occurring non-native invasive plant species on old-field succession [J]. Forest Ecology and Management, 2014, 324: 196-204. DOI: 10.1016/j.foreco.2013.10.031.
[36] SHEN W S, GAO N, MIN J, SHI W M, HE X H, LIN X G. Influences of past application rates of nitrogen and a catch crop on soil microbial communities between an intensive rotation [J]. Acta Agriculturae Scandinavica, 2016, 66(2): 97-106. DOI: 10.1080/09064710.2015.1072234.
[37] FIERER N, JACKSON R B. The diversity and biogeography of soil bacterial communities [J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(3): 626-631. DOI: 10.1073/pnas.0507535103.
[38] WU X, WU H, YE J Y, ZHONG B. Study on the release routes of allelochemicals from Pistia stratiotes Linn., and its anti-cyanobacteria mechanisms on Microcystis aeruginosa [J]. Environmental Science and Pollution Research, 2015, 22: 18994-19001. DOI: 10.1007/s11356-015-5104-4.
[39] KULMATISKI A, BEARD K H, STEVENS J R, COBBOLD S M. Plant-soil feedbacks: A meta-analytical review [J]. Ecology Letters, 2008, 11(9): 980-992. DOI: 10.1111/j.1461-0248.2008.01209.x.
[40] LINDSAY E A, COLLOFF M J, GIBB N L, WAKELIN S A. The abundance of microbial functional genes in grassy woodlands is influenced more by soil nutrient enrichment than by recent weed invasion or livestock exclusion [J]. Applied and Environmental Microbiology, 2010, 76(16): 5547-5555. DOI: 10.1128/AEM.03054-09.
[41] WAKELIN S A, GREGG A L, SIMPSON R J, LI G D, RILEY I T, MCKAY A C. Pasture management clearly affects soil microbial community structure and N-cycling bacteria [J]. Pedobiologia, 2009, 52(4): 237-251. DOI: 10.1016/j.pedobi.2008.10.001.
[42] BELNAP J, PHILLIPS S L. Soil biota in an ungrazed grassland: response to annual grass (Bromus tectorum) invasion [J]. Ecological Applicationsl, 2001, 11(5): 1261-1275. DOI: 10.1890/1051-0761(2001)011[1261: SBIAUG]2.0.CO;2.
[43] WANG C Y, XIANG J G, DU D L. The ecological effects of two invasive plants on soil microorganism community in rhizosphere [J]. Ecology and Environmental Sciences, 2012, 21(7): 1247-1251. DOI: 10.16258/j.cnki.1674-5906. 2012.07.022. (in Chinese)
[44] BARANTAL S, ROY J, FROMIN N, SCHIMANN H, HATTENSCHWILER S. Long-term presence of tree species but not chemical diversity affect litter mixture effects on decomposition in a neotropical rainforest [J]. Oecologia, 2011, 167(1): 241-252. DOI: 10.1007/s00442-011-1966-4.
[45] CHAPMAN S K, NEWMAN G S, HART S C, SCHWEITZER J A, KOCH G W. Leaf litter mixtures alter microbial community development: mechanisms for non-additive effects in litter decomposition [J]. PLoS One, 2013, 8(4): e62671. DOI: 10.1371/journal.pone.0062671.
[46] RODRIGUEZ-LOINAZ G, ONAINDIA M, AMEZAGA I, MIJANGOS I, GARBISU C. Relationship between vegetation diversity and soil functional diversity in native mixed-oak forests [J]. Soil Biology & Biochemistry, 2008, 40(1): 49-60. DOI: 10.1016/j.soilbio.2007.04.015.
[47] EISENHAUER N, BEβLER H, ENGELS C, GLEIXNER G, HABEKOST M, MILCU A, PARTSCH S, SABAIS A C W, SCHERBER C, STEINBEISS S, WEIGELT A, WEISSER W W, SCHEU S. Plant diversity effects on soil microorganisms support the singular hypothesis [J]. Ecology, 2010, 91(2): 485-496. DOI: 10.1890/08-2338.1.
(Edited by ZHENG Yu-tong)
中文导读
一年蓬和加拿大一枝黄花共同入侵对土壤细菌群落丰度的协同作用
摘要:一些入侵植物成功入侵的主因之一就是它们促进其根际土壤微生物群落的演替。此外,同一生态系统中可能遭受两种甚至是两种以上入侵植物的入侵。所以,探究共同入侵的入侵植物对土壤细菌群落的影响对阐明入侵植物成功入侵的机理具有重要作用。本文以高通量测序技术为手段,探究不同入侵程度的一年蓬和加拿大一枝黄花共同入侵对土壤细菌群落结构的影响。一年蓬和/或加拿大一枝黄花入侵显著增加了土壤细菌的、Shannon指数和Chao1指数。低度入侵的加拿大一枝黄花以及一年蓬和加拿大一枝黄花共同入侵显著增加了土壤细菌的ACE指数。一年蓬和/或加拿大一枝黄花可通过增加土壤细菌群落的多样性和丰度促进了其进一步的入侵进程,高的土壤细菌群落多样性和丰度可提升土壤功能和营养获取水平。与一年蓬和加拿大一枝黄花单一入侵相比,一年蓬和加拿大一枝黄花共同入侵显著增加了土壤细菌的ACE指数。因此,一年蓬和加拿大一枝黄花共同入侵对影响土壤细菌群落丰度具有协同作用,构建了一个利于其自身生长及其进一步入侵的土壤微环境条件。
关键词:共同入侵;一年蓬;入侵植物;土壤细菌群落;加拿大一枝黄花
Foundation item: Project(31300343) supported by the National Natural Science Foundation of China; Project(PCRRF19009) supported by Open Science Research Fund of State Key Laboratory of Pollution Control and Resource Reuse (Tongji University), China; Project supported by Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment, China
Received date: 2011-02-17; Accepted date: 2020-03-16
Corresponding author: WANG Cong-yan, PhD, Associate Professor; Tel: +86-511-88790955; E-mail: liuyuexue623@163.com; ORCID: 0000-0002-6132-3319

