
Isothermal section at 927 ℃ of Cr-Ni-Ti system
TAN Yong-heng(谭永恒), XU Hong-hui(徐洪辉), DU Yong(杜 勇)
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 18 April 2006; accepted 29 June 2007
Abstract: The isothermal section at 927 ℃ of the Cr-Ni-Ti system was established using a high-efficiency diffusion couple approach, supplemented with eight equilibrated alloys. The alloy compositions were selected on the basis of the experimental results from the diffusion couple. Both the diffusion couple specimens and the alloys were examined by means of optical microscopy, scanning electron microscopy, and electron probe microanalysis. No ternary compound is found at 927 ℃. The following five three-phase equilibria are well determined: TiNi3+(Cr)+(Ni), TiNi3+(Cr)+TiNi, TiNi+(Cr)+Cr2Ti, Ti2Ni+Cr2Ti+TiNi and Ti2Ni+Cr2Ti+(Ti). The solubilities of Cr in NiTi2, NiTi, and Ni3Ti are determined to be 7.5%, 14.5% and 11.4% (molar percent), respectively. α-Cr2Ti and β-Cr2Ti dissolve about 9.2% and 13.9% Ni (molar percent), respectively.
Key words: Cr-Ni-Ti ternary system; phase diagram; diffusion; EPMA
1 Introduction
The Cr-Ni-Ti system is a subsystem in our research project[1-7] aiming at establishing a thermodynamic database for the C-Cr-Ni-Si-Ti quinary system. Ni-Cr based alloys are important corrosion-resistant high- temperature materials, and the addition of Ti can enhance the comprehensive properties of the alloys. The Ni-based superalloys that contain Cr and Ti are currently used to manufacture parts of aircraft engines and land-based turbines. Knowledge of the phase equilibria of the Cr-Ni-Ti system is of fundamental interest in developing the new Ni-based or Ti-based high-temperature structural materials.
TAYLOR and FLOYD[8] first investigated the Ni-rich isothermal section of the system at 750, 1 000 and 1 150 ℃ by means of X-ray diffraction(XRD) and optical microscopy methods. Later, KAUFMAN and NESOR[9] performed a preliminary thermodynamic optimization for this ternary system. They calculated a number of partial isotherms at 1 027, 1 277 and 1 352 ℃. A complete isothermal section at 850 ℃ was determined by BEEK et al[10] using diffusion couples and equilibrated alloys. The samples were examined via XRD, electron probe microanalysis(EPMA) and polarized light microscopy. XU and JIN[11] measured the partial isothermal section at 927 ℃ using a diffusion triple technique. But most of the phase equilibrium relationships reported were presented with uncertainties, as the scale of some phases in the diffusion triple was too small to accurately determine the phase equilibria and some of the phase boundaries was hard to discern.
The diffusion couple technique is an efficient approach to determine ternary isothermal sections[12], but in most of cases, equilibrated alloys are desirable in order to obtain more accurate phase equilibrium data or substantiate ones of the phase equilibria. The objective of the present work is to accurately determine the phase diagram of the Cr-Ni-Ti system at 927 ℃ using four diffusion couples and key ternary alloys.
2 Experimental
Chromium (99.95%, mass percent), nickel (99.98%, mass percent) and titanium (99.97%, mass percent) were used as staring materials. Three binary alloys, Cr15Ni85, Cr36Ni64 and Cr18Ti82 (molar percent, %), were melted in an arc furnace (WKDHLI, Opto-electronics Co. Ltd. Beijing, China) under high purity argon atmosphere using a non-consumable tungsten electrode. The ingots of the alloys were remelted five times to improve their homogeneity. The mass loss of the alloys during melting was less than 0.1%. Slices of 4 mm×4 mm×12 mm were cut from the ingots. Four different diffusion couples of 4 mm×8 mm×12 mm, viz. Cr15Ni85/Ti, Cr36Ni64/Ti, Cr18Ti82/Ni and Cr36Ni64/Cr18Ti82, were made with the technique described in detail elsewhere[13]. The specimens were annealed in an L4514-type diffusion furnace (Qingdao Instrument and Equipment Co. Ltd., China) at 927 ℃ for 192 h, and then rapidly quenched in water.
After standard metallographic preparation, the microstructural investigations of the Cr-Ni-Ti couples were carried out using optical microscopy (Leica DMLP, Germany) and scanning electron microscopy (JSM- 6360LV, JEOL, Japan). The composition-distance curves for each element were determined using EPMA (JXA-8800R, JEOL, Japan). The microprobe measurements were performed perpendicular to the interfaces between every two adjacent phases in the diffusion couples. The equilibrium compositions of each phase were obtained by tangential extrapolating the composition-distance curves for each element to the phase boundaries[14].
Eight ternary alloys, the nominal compositions of which are listed in Table 1, were selected on the basis of the experimental results obtained with EPMA measurement of the diffusion couples to more accurately determine some of the phase equilibria. The alloys that were prepared by arc melting were annealed at 927 ℃ for 240 h, and then water-quenched. The metallographic samples were first examined using optical microscopy, and then analyzed using EPMA.
Table 1 Nominal compositions of Cr-Ni-Ti ternary alloys (molar percent, %)
3 Results and discussion
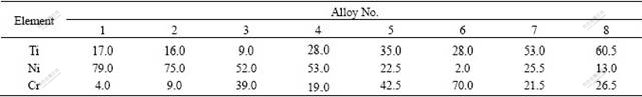
The microstructures of the diffusion couples that are annealed at 927 ℃ for 192 h are shown in Fig.1. The backscattered electron(BSE) images of the ternary alloys annealed at 927 ℃ for 240 h are presented in Fig.2.
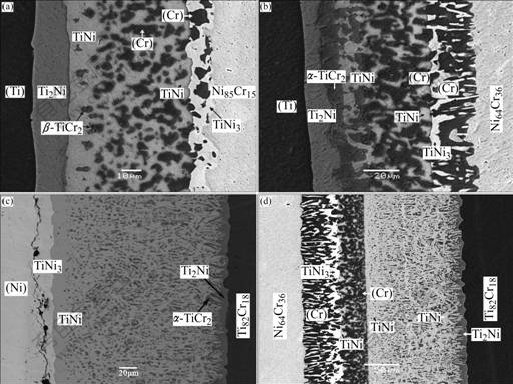
Fig.1 BSE images of four diffusion couples annealed at 927 ℃ for 192 h: (a) (Cr-85Ni)/Ti; (b) (Cr-64Ni)/Ti; (c) (Cr-82Ti)/Ni;(d) (Cr-64Ni)/(Cr-82Ti)
Fig.2 BSE images of three representative alloys annealed at 927 ℃ for 240 h: (a) Alloy 3 (Cr39.0Ni52.0Ti9.0); (b) Alloy 4 (Cr19.0Ni53.0Ti28.0); (c) Alloy 5 (Cr42.5Ni22.5Ti35.0).
After interdiffusion at 927 ℃, the sequence of the phases that form in the diffusion couple of (Cr-85Ni)/Ti is (Ti)→Ti2Ni→TiNi+b-TiCr2→TiNi3+(Cr)→(Cr-85Ni),as shown in Fig.1(a). The phases b-TiCr2 and (Cr) are scattered in the phase zones of TiNi and TiNi3, respectively. And there are three-phase conjunctions of TiNi, (Cr) and TiNi3 at the interface between TiNi and TiNi3. The sequence of the phases in the diffusion couple (Cr-64Ni)/Ti, as shown in Fig.1(b), is (Ti)→Ti2Ni→α-TiCr2→TiNi+β-TiCr2→TiNi+(Cr)→ TiNi3→(Cr)→(Cr-64Ni). There appear two three-phase conjunctions of β-TiCr2+TiNi+(Cr) and TiNi+(Cr)+TiNi3 in the interdiffusion zone.
The situation for the diffusion couple (Cr-82Ti)/Ni is similar to that for the diffusion couple (Cr-85Ni)/Ti. The sequence of the phases in this diffusion couple is (Ni)→TiNi3→TiNi+α-TiCr2→Ti2Ni→(Cr-82Ti), and α-TiCr2 is scattered in the phase zone of TiNi, as shown in Fig.1(c). The sequence of the phases in the diffusion couple (Cr-64Ni)/(Cr-82Ti) is (Cr-64Ni)→(Cr)→TiNi3 →(Cr)+TiNi→TiNi+Ti2Ni→Ti2Ni→(Cr-82Ti), as shown in Fig.1(d). The microstructural complexity in the above four diffusion couples may be ascribed to the inhomogeneity of the as-cast alloys used as diffusion couple end-members, which leads to the appearance of the two-phase co-existing zones and three-phase conjunctions in the diffusion couples.
As shown in Fig.2, the three representative ternary alloys, (Cr39.0Ni52.0Ti9.0), (Cr19.0Ni53.0Ti28.0) and (Cr42.5Ni22.5Ti35.0), are located in the three-phase regions TiNi3+(Cr)+(Ni), TiNi3+(Cr)+TiNi, and TiNi+(Cr)+ β-TiCr2, respectively.
The tie-line data and the tie-triangle data obtained with EPMA measurement of the Cr-Ni-Ti diffusion couples and ternary alloys are listed in Tables 2 and 3, respectively. Using these experimental data, the isothermal section at 927 ℃ is constructed in Fig.3. The presently obtained data on the binary systems Cr-Ti and Ni-Ti are in agreement with the literature data[15-16], respectively. One of the three-phase equilibria, TiNi+ α-TiCr2+β-TiCr2, is not well determined and presented in dashed lines, which is due to the fact that the two phases, α-TiCr2 and b-TiCr2, are very difficult to identify.
Table 2 Tie-line data on phase equilibria of Cr-Ni-Ti system at 927 ℃1
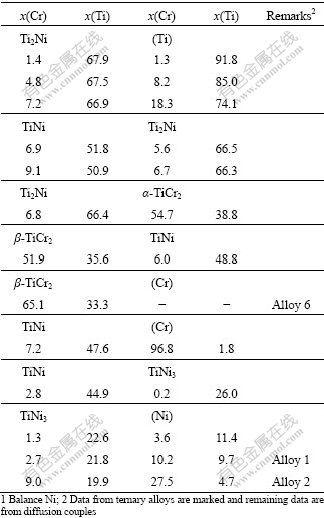
Table 3 Tie-triangle data on phase equilibria of Cr-Ni-Ti system at 927 ℃1
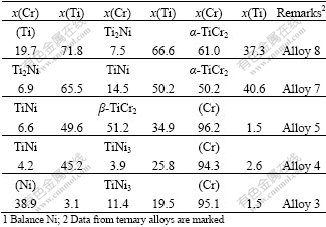
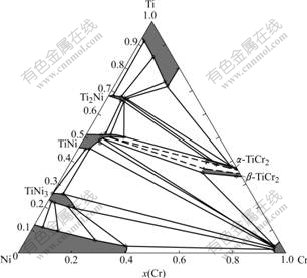
Fig.3 Isothermal section of Cr-Ni-Ti system at 927 ℃
According to the EPMA data, the solubilities of Cr in NiTi2, NiTi, and Ni3Ti are determined to be 7.5%, 14.5% and 11.4% (molar percent), respectively. And the solubilities of Ni in α-TiCr2 and β-TiCr2 are measured to be 9.2% and 13.9% (molar percent), respectively.
4 Conclusions
1) The isothermal section at 927?C of the Cr-Ni-Ti system is established using a high-efficiency diffusion couple approach with the aid of eight equilibrated alloys. It is obvious that the phase equilibrium data determined in the present work are more accurate than these previously reported.
2) No ternary compound is found at 927 ℃. The following five three-phase equilibria are observed: TiNi3+(Cr)+(Ni), TiNi3+(Cr)+TiNi, TiNi+(Cr)+β-Cr2Ti, Ti2Ni+α-Cr2Ti+TiNi and Ti2Ni+α-Cr2Ti+(Ti). The solubilities of Cr in NiTi2, NiTi, and Ni3Ti are determined to be 7.5%, 14.5% and 11.4% (molar percent), respectively. α-TiCr2 and β-TiCr2 dissolve about 9.2% and 13.9% Ni (molar percent), respectively.
3) It is demonstrated that the diffusion couple approach is powerful in determining ternary phase diagrams, and useful especially in conjunction with the equilibrated alloys method.
References
[1] DU Yong, SCHUSTER J C. Experimental investigation and thermodynamic modeling of the Ni-Ti-C system [J]. Zeitschrift Für Metallkunde, 1998, 89(6): 399-410.
[2] SCHUSTER J C, DU Yong. Thermodynamic description of the system Ti-Cr-C [J]. Calphad: Computer Coupling of Phase Diagrams and Thermochemistry, 1999, 23(3/4): 393-408.
[3] DU Yong, SCHUSTER J C. Experimental investigations and thermodynamic descriptions of the Ni-Si and C-Ni-Si systems [J]. Metallurgical and Materials Transactions A, 1999, 30(9): 2409-2418.
[4] SCHUSTER J C, DU Yong. Experimental investigation and thermodynamic modeling of the Cr-Ni-Si system [J]. Metallurgical and Materials Transactions A, 2000, 31(7): 1795-1803.
[5] DU Yong, SCHUSTER J C, SEIFERT H J, ALDINGER F. Experimental investigation and thermodynamic calculation of the titanium-silicon- carbon system [J]. Journal of the American Ceramic Society, 2000, 83(1): 197-203.
[6] DU Yong, SCHUSTER J C, PERRING L. Experimental investigation and thermodynamic description of the constitution of the ternary system Cr-Si-C [J]. Journal of the American Ceramic Society, 2000, 83(8): 2067-2073.
[7] DU Yong, SCHUSTER J C. Experimental investigation and thermodynamic description of the Cr-Si-Ti system [J]. Scandinavian Journal of Metallurgy, 2002, 31(1): 25-31.
[8] TAYLOR A, FLOYD R W. The constitution of Ni-rich alloys of the Ni-Cr-Ti system [J]. The Journal of the Institute of Metals, 1952, 80: 577-587.
[9] KAUFMAN L, NESOR H. Calculation of superalloy phase diagrams EM DASH 1 [J]. Metallurgical Transactions, 1974, 5(7): 1617-1621.
[10] VAN BEEK J A, KODENTSOV A A, VAN LOO F J J. Phase equilibria of the Cr-Ni-Ti system at 850 ℃ [J]. Journal of Alloys and Compounds, 1998, 270: 151-155.
[11] XU Hong-hui, JIN Zhan-peng. The determination of the isothermal section at 1 200 K of the Cr-Ni-Ti phase diagram [J]. Scripta Materialia, 1997, 37(2): 147-150.
[12] KODENTSOV A A, BASTIN G F, VAN LOO F J J. The diffusion couple technique in phase diagram determination [J]. Journal of Alloys and Compounds, 2001, 320: 207-217.
[13] XU Hong-hui, DU Yong, TANG Cheng-ying, HE Yue-hui, HUANG Bai-yun, LI Shi-tong. Isothermal section at 950 ℃ of the Co-Nb-Ti system [J]. Materials Science and Engineering A, 2005, 412: 336-341.
[14] XU Hong-hui, DU Yong, Tan Yong-heng,HE Yue-hui, LI Shi-tong, ZHANG Xiang. Phase equilibria of the Co-Ni-Ta system at 1 100 ℃ [J]. Journal of Alloys and Compounds, 2006, 425(1/2): 153-158.
[15] ZHUANG Wei-dong, SHEN Jian-yun, LIU Yu-qin, LING Ling, SHANG Shun-li, DU Yong, SCHUSTR J C. Thermodynamic optimization of the Cr-Ti system [J]. Zeitschrift Für Metallkunde, 2000, 91(2): 121-127.
[16] BELLEN P, KUMAR K C HARI, WOLLANTS P. Thermodynamic assessment of the Ni-Ti phase diagram [J]. Zeitschrift Für Metallkunde, 1996, 87(12): 972-978.
Foundation item: Project(50425103) supported by the National Science Fund for Distinguished Young Scholars of China; Project(50571114) supported by the National Natural Science Foundation of China
Corresponding author: DU Yong; Tel: +86-731-8836213; fax: +86-731-8710855; E-mail: yong-du@mail.csu.edu.cn
(Edited by YANG Bing)