文章编号:1004-0609(2016)-04-0901-07
卤素离子催化作用下SO2还原沉金后液及其热力学特征
马亚赟1, 2,郑雅杰2,丁光月1,王俊文1,董俊斐2,张福元2
(1. 太原理工大学 化学化工学院,太原 030024;
2. 中南大学 冶金与环境学院,长沙 410083)
摘 要:以沉金后液为原料,采用SO2为还原剂,研究在卤素以及卤素复合催化剂存在条件下还原沉金后液中的硒碲以及捕集贵金属金铂钯的工艺。结果表明:在85 ℃、硫酸浓度167 g/L、SO2流量0.2 L/min的条件下,当Cl-催化剂浓度为1.1 mol/L、反应时间2 h,或当Br-催化剂浓度为0.5 mol/L、反应时间3 h,或当I-催化剂浓度为0.3 mol/L、反应时间2 h时,硒金铂钯还原率达到100%,碲还原率达到99.60%以上。采用卤素复合催化剂,当NaCl与NaBr摩尔比为1:2时,有助于加快硒碲的还原,而且降低了催化剂的用量。SO2催化还原后产物中含碲74.56%、铜11.85%、硒7.38%,贵金属金3.89%、铂0.19%、钯1.02%(质量分数);还原产物中碲以单质状态存在,产物形貌为球状体。热力学分析表明:硫酸浓度为167 g/L时,Se(Ⅳ)主要以H2SeO3形式存在,Te(Ⅳ)主要以H3TeO3+形式存在;当溶液中有Cl-存在时,溶液中H3TeO3+在Cl-缔合作用下逐渐转变为TeCl62-,其电极电势较H3TeO3+的正,促进碲的还原。
关键词:SO2;还原;卤素离子;沉金后液;热力学
中图分类号:TF09 文献标志码:A
铜阳极泥是铜电解精炼过程中产出的一种副产品,它是由阳极铜在电解精炼过程中不溶于电解液的各种物质所组成,一般含有大量的贵金属及稀有金属,特别是金银铂钯等贵金属,使铜阳极泥的价值倍增,是提取贵金属及稀散金属的重要原料[1-12]。目前,国内采用湿法流程处理铜阳极泥的工厂已达40%以上,铜阳极泥经预处理[13-16]脱铜之后,采用亚硫酸钠或氨水浸出银、氯酸钠浸出金工艺分别得到分银液和沉金液,分银液用水合肼或甲醛还原得到银粉,沉金液用亚硫酸钠或草酸还原得到金粉和沉金后液。
目前的工艺一般是将锌粉[17-19]作为还原剂加入沉金后液中置换其中的稀贵元素得到硒碲精矿。锌粉置换法作为一种传统工艺流传至今,因其工艺简单、操作方便受到人们的青睐。但是,锌粉几乎不还原硒碲,得到的稀贵元素精矿中贵金属的品位低,给下游工艺回收金铂钯造成困难。已有研究[20-22]采用SO2还原法或者Na2SO3还原法处理沉金后液,取得较好的效果。本文作者在此基础上,采用SO2复合催化法还原沉金后液回收硒碲捕集金铂钯,重点研究了单一卤素离子催化剂以及卤素复合催化剂催化还原的特征,为工业生产指明方向。
1 实验
1.1 实验原料
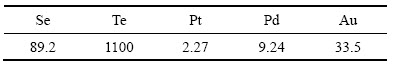
实验原料为某铜冶炼厂的沉金后液,其主要成分如表1所列。
表1 沉金后液主要化学成分
Table 1 Main chemical compositions of precipitated gold solution (mg/L)
1.2 实验步骤
准确量取一定量的沉金后液倒入三颈瓶中,将三颈瓶固定于电加热套中,启动搅拌,加浓硫酸调整酸度,将电加热套加热至一定温度,并保持温度不变,加入一定量催化剂和还原剂,反应一段时间后过滤,滤液定容分析,滤渣干燥称取质量,其工艺流程图如图1所示。
1.3 分析与检测
采用美国热电元素公司生产的Intrepid II XSP型电感耦合等离子体发射光谱仪(ICP)分析溶液成分;采用X射线荧光光谱仪(XRF)定性半定量分析固体物质成分;采用日本理学生产的D/max-TTR III型X射线衍射仪(XRD)分析固体物质物相;日本电子株式会社生产的JSM-6300型扫描电镜(SEM)观察固体产物微观形貌。
溶液中物质还原率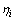 按下式计算:
按下式计算:
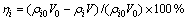 (1)
(1)
式中: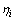 为第i种元素的还原率;
为第i种元素的还原率;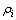 为反应后滤液中第i种元素的质量体积浓度,mg/L;V为反应后滤液体积,L;
为反应后滤液中第i种元素的质量体积浓度,mg/L;V为反应后滤液体积,L;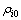 为沉金后液中第i种物质的质量体积浓度,mg/L;V0为实验用沉金后液体积,L。
为沉金后液中第i种物质的质量体积浓度,mg/L;V0为实验用沉金后液体积,L。
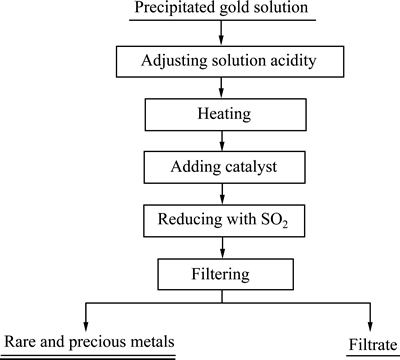
图1 沉金后液回收硒碲精矿工艺流程
Fig. 1 Flow sheet of recovering selenium and tellurium concentrate from precipitated gold solution
2 结果与讨论
2.1 Cl-浓度对稀贵金属还原率的影响
准确量取200 mL沉金后液,根据计算,完全还原沉金后液中的稀贵金属离子所需SO2,其流量应控制在0.2 L/min左右,控制反应温度85 ℃、体系硫酸浓度为167 g/L、反应时间2 h时,考察Cl-浓度对稀贵金属还原率的影响,其结果如图2所示。
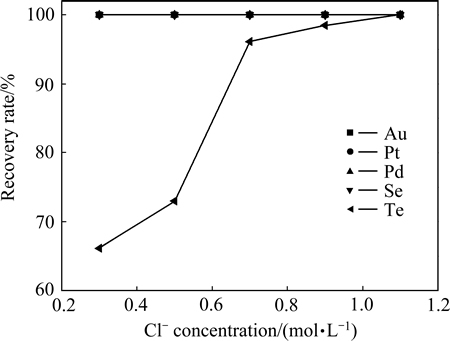
图2 Cl-浓度对稀贵元素还原率的影响
Fig. 2 Effect of Cl- concentration on recovery rate of precious metals
由图2可知,在此催化剂浓度范围内,沉金后液中Se、Au、Pt、Pd均被全部还原,还原率为100%。碲的还原率随Cl-浓度增加而增加,当Cl-浓度由0.3 mol/L增加到1.1 mol/L时,碲的还原率由68.26%增加到99.60%。
在硫酸体系中,碲以硫酸氧碲的形式存在。Cl-的催化作用在于改变溶液中离子的存在形态,当体系中有氯离子存在时,溶液中的碲发生如下变化:
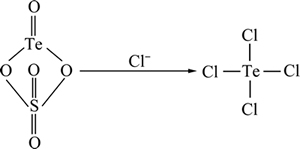 (2)
(2)
Cl-的存在破坏了碲氧双键的稳定性,同时消除了硫酸根离子的阻隔效应,从而降低反应的活化能,有利于还原反应的进行[21]。反应式如下所示:
TeCl4+2SO2+2H2O=Te↓+2HSO4-+2H++4Cl- (3)
SeCl4+2SO2+2H2O=Se↓+2HSO4-+2H++4Cl- (4)
2AuCl4-+3SO2+6H2O=2Au↓+3HSO4-+9H++8Cl- (5)
PtCl62-+2SO2+2H2O=Pt↓+2HSO4-+2H++6Cl- (6)
PdCl62-+2SO2+2H2O=Pd↓+2HSO4-+2H++6Cl- (7)
2.2 Br-催化剂体系中反应时间对稀贵金属还原率的影响
当反应温度为85 ℃、体系硫酸浓度为167 g/L、SO2流量为0.2 L/min、Br-浓度为0.5 mol/L时,反应时间对还原率的影响如图3所示。
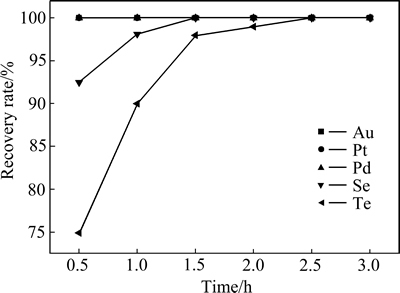
图3 Br-体系中时间对稀贵元素还原率的影响
Fig. 3 Effect of reaction time on recovery rate of precious metals in Br- system
金、铂、钯在0.5 h以内全部还原,还原率为100%;当反应时间从0.5 h增加到3 h时,碲和硒的还原率随反应时间的延长而增加,硒的还原率由91.74%增加到100%,碲的还原率由74.82%增加到99.94%。
Br-催化机理与Cl-的相似,不同的是Br-作为催化剂时,其用量可以明显较少。Br-首先破坏TeOSO4中非常稳定的碲氧双键,碲离子与空间位阻较大的硫酸根离子分离,从而降低反应的活化能,反应式如下所示:
2NaBr+H2SO4=Na2SO4+2HBr (8)
4HBr+TeOSO4=TeBr4+H2SO4+H2O (9)
TeBr4+2H2SO3+2H2O=Te+2H2SO4+4HBr (10)
2.3 I-催化剂体系中稀贵金属的还原
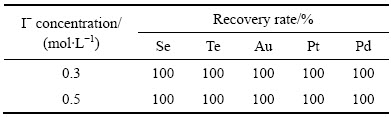
当反应温度为85 ℃、反应时间为2 h、体系中的硫酸浓度为167 g/L、SO2流量为0.2 L/min、I-浓度分别为0.3 mol/L和0.5 mol/L时,沉金后液中的稀贵金属的还原率如表2所列。
由表2可知,I-浓度为0.3 mol/L和0.5 mol/L时,稀贵金属均已被完全还原,还原率为100%。当I-浓度为0.5 mol/L时,除了生成铅黑色稀贵元素粉末外还有团块状固体生成,主要是碘过量,被氧化成单质造成;当I-浓度降低到0.3 mol/L时,团块状固体消失,同时,各种元素的还原率达到100%。因此,I-浓度为0.3 mol/L是比较合理的催化剂用量,进一步降低了催化剂用量。
表2 I-催化剂体系中稀贵金属的还原率
Table 2 Recovery rate of rare and precious metals in I- system
各卤素催化剂还原后产物如图4所示。由图4可知,I-催化机理与其他卤素的不同,由于I-半径较大,很容易失去最外层电子还原成单质碘。溶液中加入I-后,I-与其中的稀贵元素反应生成I2,碘单质被亚二氧化硫还原成HI,HI还原TeOSO4生成TeI而不生成碲单质。实验过程中铅黑色粉末洗涤会溶解证明了还原产物并非单质,其反应方程式如下所示:
SO2+2H2O+I2=H2SO4+2HI (11)
8HI+2TeOSO4=2TeI+3I2+2H2SO4+2H2O (12)
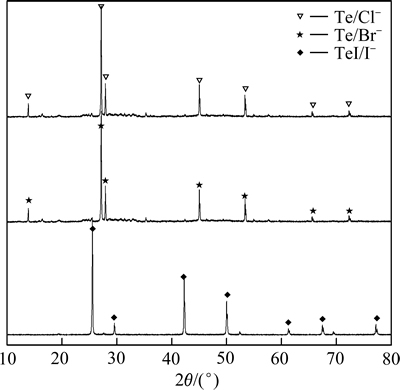
图4 不同卤素催化剂作用下SO2还原产物XRD谱
Fig. 4 XRD patterns of products reduced by SO2 with different halogen catalysts
2.4 复合催化剂体系中稀贵金属的还原
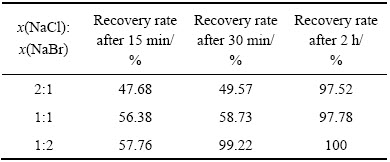
当反应温度为85 ℃、反应时间为2 h、体系中的硫酸浓度为167 g/L、SO2流量为0.2 L/min时,采用复合催化剂还原,复合催化剂的成分为NaCl和NaBr,在体系中的总浓度为0.3 mol/L,实验中的变量为NaCl和NaBr的摩尔比,3组实验中NaCl和NaBr摩尔比分别为2:1、1:1和1:2。实验结果分别如表3和表4所示。
由表3可知,当x(NaCl):x(NaBr)=1:2时,Se的还原速度最快。由表4可知,当x(NaCl):x(NaBr)=1:2时,Te的还原速度最快,由此可知,随着复合催化剂中NaBr含量增加,硒碲的还原速率加快。对x(NaCl): x(NaBr)=1:2复合催化还原2 h后的溶液进行检测发现,金铂钯被全部还原,还原率为100%,对还原反应2 h所得产物进行XRF荧光衍射定性分析检测成分如表5所列,对反应所得产物进行X衍射和表面电子扫描电镜(SEM)分析,其实验结果分别如图5和6所示。
表3 不同摩尔比的催化剂组分对Se还原率的影响
Table 3 Effects of different mole fractions of catalyst component on recycling selenium
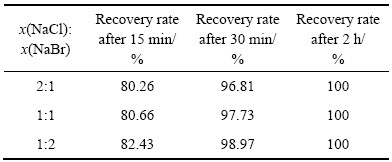
表4 不同摩尔比的催化剂组分对Te还原率的影响
Table 4 Effects of different mole fractions of catalyst component on recycling tellurium
由表5可知,稀散金属碲含量为74.56%,硒为7.38%,贵金属金铂钯含量分别为3.89%、0.19%、1.02%,总量为5.1%,贵金属得到富集,此外铜占11.85%,镁占0.21%,铟占0.24%,其他杂质占0.66%(质量分数)。由图5可知,还原产物主要是碲单质,由图6可知,所得产物形貌呈球状体。
表5 还原产物XRF定性分析结果
Table 5 XRF analysis of reduction product (mass fraction, %)
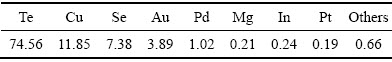
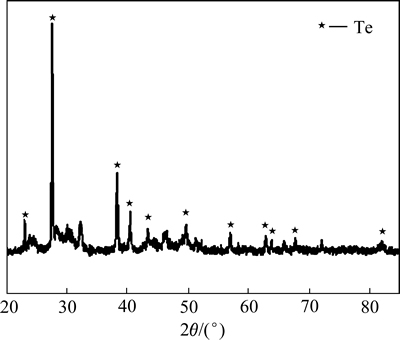
图5 还原产物的XRD谱
Fig. 5 XRD pattern of reduction product

图6 还原产物的SEM像
Fig. 6 SEM image of reduction product
综合以上结果可知,当复合催化剂中的NaBr摩尔分数增高,NaCl摩尔分数减小时,反应速度快,催化效果好,也就是说当NaBr和NaCl作为还原沉金后液的催化剂时NaBr更有效。另外与单独使用NaCl作为催化剂相比,复合催化剂能够明显减少催化剂的用量。
2.5 硫酸体系下SO2还原稀贵金属的热力学特征
当反应温度为85 ℃、反应时间为2 h、SO2流量为0.2 L/min、体系中的Cl-浓度为1.1 mol/L时,硫酸浓度对稀贵金属还原率的影响如图7所示。
由图7可知,在此浓度范围内,沉金后液中的金和铂被全部还原,还原率为100%;当硫酸浓度从46 g/L增加到368 g/L时,碲的还原率随体系中硫酸浓度增加而增加,由69.61%增加到100%;钯的还原率随硫酸浓度增加而增加,由93.94%增加到100%;硒的还原率随硫酸浓度的增加而增加,从91.02%增加到100%。
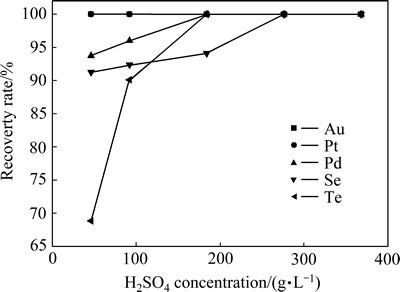
图7 硫酸浓度对稀贵金属还原率的影响
Fig. 7 Effect of H2SO4 concentration on rare metals reduction rate
沉金后液中Se(Ⅳ)和Te(Ⅳ)可能存在形式主要有SeO32-、HSeO3-、H2SeO3和TeO32-、HTeO3-、H2TeO3、H3TeO3+等形态,其热力学关系式[23-25]为
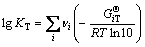 (13)
(13)
式中:KT为反应热力学平衡常数;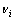 为化学计量系数;
为化学计量系数;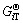 为反应各物质吉布斯自由能。根据式(13)及c[M(Ⅳ)]T=c(MO32-)+c(HMO3-)+c(H2MO3) +c(H3MO3+),其中c[M(Ⅳ)]T指溶液中M4+的总浓度及M指硒或碲,可得25 ℃时沉金后液中Se(Ⅳ)和Te(Ⅳ)的不同形态组分浓度对pH的关系图,如图8和9所示。
为反应各物质吉布斯自由能。根据式(13)及c[M(Ⅳ)]T=c(MO32-)+c(HMO3-)+c(H2MO3) +c(H3MO3+),其中c[M(Ⅳ)]T指溶液中M4+的总浓度及M指硒或碲,可得25 ℃时沉金后液中Se(Ⅳ)和Te(Ⅳ)的不同形态组分浓度对pH的关系图,如图8和9所示。
由图8可知,pH≤2时,Se(Ⅳ)主要以H2SeO3形态存在;由图9可知,pH≤2时,Te(Ⅳ)主要以H3TeO3+形态存在。当沉金后液中硫酸浓度为167 g/L时,c(H+)=3.408 mol/L,即pH<0,由图8和9可知,此时沉金后液中SeO32-、HSeO3-、TeO32-、HTeO3-和H2TeO3含量很少,Se(Ⅳ)主要以H2SeO3形式存在,Te(Ⅳ)主要以H3TeO3+形式存在。
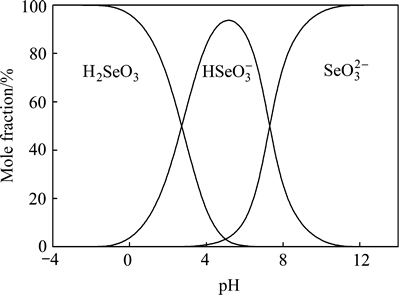
图8 Se(Ⅳ)浓度组分-pH图
Fig. 8 Curves of Se(Ⅳ) concentration-pH
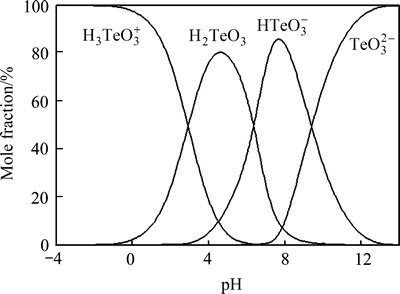
图9 Te(Ⅳ)浓度组分-pH图
Fig. 9 Curves of Te(Ⅳ) concentration-pH
对于含硫酸的沉金后液体系,可能存在的离子及化合物的标准电极电位如表6[26]所列。
表6 电极反应及标准电极电势[26]
Table 6 Electrode reaction and standard electrode potential[26]
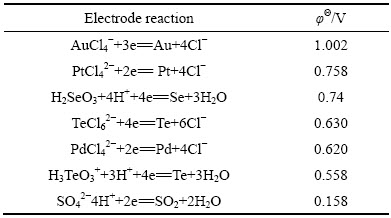
由表6可知,在沉金后液中金的还原电极电势最高为1.002 V,最易被SO2还原,H2SeO3在酸性条件下易被还原,H3TeO3+相对金铂钯氧化性低,相应还原率较低。当溶液中有Cl-存在时,溶液中H3TeO3+在Cl-缔合作用下逐渐转变为TeCl62-,还原电极电势TeCl62-的较H3TeO3+的正,从而促进溶液中Te(Ⅳ)的还原;当溶液中酸度越高时,H3TeO3+也就越容易形成,促进其与Cl-的反应,以此形成良性循环,有利于增加Te回收率。
3 结论
1) 采用SO2还原沉金后液,Cl-催化剂最佳浓度为1.1 mol/L,Br-催化剂最佳浓度为0.5 mol/L,I-催化剂最佳浓度为0.3 mol/L,复合催化剂NaCl与NaBr总浓度为0.3 mol/L,摩尔比x(NaCl):x(NaBr)=1:2。
2) 采用SO2还原沉金后液,反应温度85 ℃、反应时间2 h、体系中的硫酸浓度167 g/L、SO2流量0.2 L/min、复合催化剂(NaCl和NaBr)在体系中的总浓度为0.3 mol/L,其中摩尔比x(NaCl):x(NaBr)=1:2时,硒、碲、金、铂、钯的还原率均为100%。
3) XRF分析表明:还原产物中碲、硒、金、铂、钯品位分别为74.56%、7.38%、3.89%、0.19%、1.02%;XRD分析表明:还原产物中碲以单质状态存在;SEM分析表明:还原产物为球状体。
4) 热力学分析表明:硫酸体系下,当硫酸浓度为167 g/L时,Se(Ⅳ)主要以H2SeO3形式存在;电极电势较高,易被还原。Te(Ⅳ)主要以H3TeO3+形式存在,电极电势较低,不易被还原,但是当溶液中有Cl-存在时,溶液中H3TeO3+在Cl-缔合作用下逐渐转变为TeCl62-,其电极电势较H3TeO3+正,促进了碲的还原。
REFERENCES
[1] 郑雅杰, 汪 蓓, 史建远, 孙召明, 刘昭成. 铜阳极泥预处理富集金银的研究[J]. 中南大学学报(自然科学版), 2010, 41(3): 865-870.
ZHENG Ya-jie, WANG Bei, SHI Jian-yuan, SUN Zhao-ming, LIU Zhao-cheng. Pretreatment of copper anode slime for concentrating gold and silver[J]. Journal of Central South University (Science and Technology), 2010, 41(3): 865-870.
[2] 郭学益, 肖彩梅, 钟菊芽, 田庆华. 铜阳极泥处理过程中贵金属的行为[J]. 中国有色金属学报, 2010, 20(5): 990-998.
GUO Xue-yi, XIAO Cai-mei, ZHONG Ju-ya, TIAN Qing-hua. Behaviors of precious metals in process of copper anode slime treatment[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(5): 990-998.
[3] 王吉坤, 张博亚. 铜阳极泥现代综合利用技术[M]. 北京: 冶金工业出版社, 2008.
WANG Ji-kun, ZHANG Bo-ya. Modern utilization technology of copper anode slime[M]. Beijing: Metallurgical Industry Press, 2008.
[4] 叶跃威, 杨建国. 用锌粉从高铜铅含氰贵液中置换金银[J]. 湿法冶金, 2007, 26(3): 150-153.
YE Yue-wei, YANG Jian-guo. Displacement of gold and silver from cyanide leaching solution containing copper and lead with zinc powder[J]. Hydrometallurgy of China, 2007, 26(3): 150-153.
[5] YASIN K, GULDEM K, SERVET T. An investigation of copper and selenium recovery from copper anode slimes[J]. International Journal of Mineral Processing, 2013, 124(1): 75-82.
[6] 李雪娇, 杨洪英, 佟琳琳, 陈国宝. 铜阳极泥的工艺矿物学[J]. 东北大学学报(自然科学版), 2013, 34(4): 560-563.
LI Xue-jiao, YANG Hong-ying, TONG Lin-lin, CHEN Guo-bao. Technological mineralogy of copper anode slime[J]. Journal of Northeastern University (Natural Science), 2013, 34(4): 560-563.
[7] 刘伟峰, 杨天足, 刘又年, 张 霖, 张杜超, 王 安. 脱除铜阳极泥中贱金属的预处理工艺[J]. 中南大学学报(自然科学版), 2013, 44(4): 1333-1337.
LIU Wei-feng, YANG Tian-zu, LIU You-nian, ZHANG Lin, ZHANG Du-chao, WANG An. Pretreatment process for removing base metals from copper anode slime[J]. Journal of Central South University (Science and Technology), 2013, 44(4): 1333-1337.
[8] 陈国宝, 杨洪英, 郭 军, 李雪娇. 铜阳极泥选冶富集金银的粗选研究[J]. 贵金属, 2013, 34(3): 32-36.
CHEN Guo-bao, YANG Hong-ying, GUO Jun, LI Xue-jiao. The rougher flotation process of copper anode slime for collecting gold and silver[J]. Precious Metals, 2013, 34(3): 32-36.
[9] ATEFEH K, SATTAR G, DARIUSH A. Ag recovery from copper anode slime by acid leaching at atmospheric pressure to synthesize silver nanoparticles[J]. International Journal of Mining Science and Technology, 2014, 24(1): 251-257.
[10] LU Dian-kun, CHANG Yong-feng, YANG Hong-ying, XIE Feng. Sequential removal of selenium and tellurium from copper anode slime with high nickel content[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(4): 1307-1314.
[11] XIONG Yong-liang. Predicted equilibrium constants for solid and aqueous selenium species to 300 ℃: Applications to selenium-rich mineral deposits[J]. Ore Geology Reviews, 2003, 23(3/4): 259-276.
[12] ZHENG Ya-jie, CHEN Kun-kun. Leaching Kinetics of selenium-tellurium-rich materials in sodium sulfite solutions[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(2): 536-543.
[13] 郑雅杰, 陈昆昆. 从溶液中回收稀贵金属的一种方法: CN, 101928834A[P]. 2010-12-29.
ZHENG Ya-jie, CHEN Kun-kun. The method of recovering rare metals from solution: CN, 101928834A[P]. 2010-12-29.
[14] 郑雅杰, 张福元, 马亚赟. 一种复合还原高效回收稀贵金属的方法: CN, 104561579A[P]. 2015-04-29.
ZHENG Ya-jie, ZHANG Fu-yuan, MA Ya-yun. The method of compound reducing and recovering rare metals efficiently: CN, 104561579A[P]. 2015-04-29.
[15] 张博亚, 王吉坤. 加压酸浸预处理铜阳极泥的工艺研究[J]. 矿冶工程, 2007, 27(5): 41-43.
ZHANG Bo-ya, WANG Ji-kun. The technological research on pre-treating copper anode slime with pressure acid leaching method[J]. Mining and Metallurgical Engineering, 2007, 27(5): 41-43.
[16] HE Shan-ming, WANG Ji-kun, XU Zhi-feng, WANG Jin-liang, GAN Lei. Removal of copper and enrichment of precious metals by pressure leaching pretreatment of copper anode slime in sulfuric acid medium[J]. Precious Metals, 2014, 35(4): 48-53.
[17] 叶跃威, 杨建国. 用锌粉从高铜铅含氰贵液中置换金银[J]. 湿法冶金, 2007, 26(3): 150-153.
YE Yue-wei, YANG Jian-guo. Displacement of gold and silver from cyanide leaching solution containing copper and lead with zinc powder[J]. Hydrometallurgy of China, 2007, 26(3): 150-153.
[18] 胡建辉. 从金还原后液中置换铂钯的工艺优化研究[J]. 湿法冶金, 2000, 19(2): 22-25.
HU Jian-hui. Study on optimum process for displacing Pt and Pd from the solution Au reduced[J]. Hydrometallurgy of China, 2000, 19(2): 22-25.
[19] 郑雅杰, 陈昆昆. 采用Na2SO3溶液从硒渣中选择性浸出Se及其动力学[J]. 中国有色金属学报, 2012, 22(2): 585-591.
ZHENG Ya-jie, CHEN Kun-kun. Selective leaching Se from selenium residue by Na2SO3 solutions and leaching kinetics[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(2): 585-591.
[20] 郑雅杰, 陈昆昆, 孙召明. SO2还原沉金后液回收硒碲及捕集铂钯[J]. 中国有色金属学报, 2011, 21(9): 2258-2264.
ZHENG Ya-jie, CHEN Kun-kun, SUN Zhao-ming. Recycling Se and Te and capturing Pt and Pd from solution after precipitating gold by SO2 reduction[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(9): 2258-2264.
[21] 孙召明, 郑雅杰. Te(Ⅳ)-H2SO4-H2O体系中卤素离子催化还原Te(Ⅳ)反应动力学[J]. 中国有色金属学报, 2010, 20(12): 2438-2444.
SUN Zhao-ming, ZHENG Ya-jie. Reaction kinetics of Te(Ⅳ) using halogen ions as catalyst in Te(Ⅳ)-H2SO4-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(12): 2438-2444.
[22] 张福元, 郑雅杰, 孙召明, 马亚赟, 董俊斐. 采用亚硫酸钠还原法从沉金后液中回收稀贵金属[J]. 中国有色金属学报, 2015, 25(8): 2293-2299.
[22]he Chinese Journal of Nonferrous Metals, precipitated gold solution by Na2SO3 reduction[]ZHANG Fu-yuan, ZHENG Ya-jie, SUN Zhao-ming, MA Ya-yun, DONG Jun-fei. Recovery of rare and precious metals from precipitated gold solution by Na2SO3 reduction[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(8): 2293-2299.
[23] SEBY F, POTIN-GAUTIER M, GIFFAUT E, BORGE G, DONARD O F X. A critical review of thermodynamic data for selenium species at 25 ℃[J]. Chemical Geology, 2001, 171(3/4): 173-194.
[24] GRUNDLER P, BRUGGER J, ETSCHMANN B E. Speciation of aqueous tellurium(Ⅳ) in hydrothermal solutions and vapors, and the role of oxidized tellurium species in Te transport and gold deposition[J]. Geochimica et Cosmochimica Acta, 2013, 120: 298-325.
[25] MOKMELI M, DREISINGER D, WASSINK B. Thermo- dynamics and kinetics study of tellurium removal with cuprous ion[J]. Hydrometallurgy, 2014, 147(148): 20-29.
[26] 吴维昌, 冯洪清, 吴开冶. 标准电极电位数据手册[M]. 北京: 科学出版社, 1991: 30-224.
WU Wei-chang, FENG Hong-qing, WU Kai-ye. Manual of standard electrode potential date[M]. Beijing: Science Press, 1991:30-224.
Precipitated gold solution reduced by SO2 under halogen ion composite catalyst and its thermodynamic characteristics
MA Ya-yun1, 2, ZHENG Ya-jie2, DING Guang-yue1, WANG Jun-wen1, DONG Jun-fei2, ZHANG Fu-yuan2
(1. College of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, China;
2. School of Metallurgical and Environmental, Central South University, Changsha 410083, China)
Abstract: Selenium and tellurium were recovered and gold, platinum and palladium were captured from precipitated gold solution. SO2 was used as reducer, halogen ion and composited halogen ions were used as catalysts in the reaction. The experimental results show that the reduction rates of selenium, gold, platinum and palladium are 100% and the reduction rate of tellurium is more than 99.60% when Cl- concentration is 1.1 mol/L and reaction time is 2 h, or Br- concentration is 0.5 mol/L and reaction time is 3 h, or I- concentration is 0.3 mol/L, reaction time is 2 h. Under the condition of reaction temperature 85 ℃, H2SO4 concentration 167 g/L and SO2 flow rate 0.2 L/min. The reaction speed is faster than that of NaCl catalyst when the molar ratio of NaCl to NaBr in the composited catalysts is 1:2. The mass fractions of tellurium, copper, selenium, gold, platinum, palladium in the reduction product are 74.56%, 11.85%, 7.38%, 3.89%, 0.19%, 1.02% (mass fraction), respectively. Tellurium exists in the form of simple substance in the reduction product and the morphologies of the product are spheroid bodies. Thermodynamic analysis show that Se(Ⅳ) mainly exists in the form of H2SeO3, Te(Ⅳ) mainly exists in the form of H3TeO3+, H3TeO3+ gradually transforms into TeCl62- in association with Cl-. Cl- promots the reduction of Te(Ⅳ) because the electrode potential of TeCl62- is higher than H3TeO3+.
Key words: SO2; reduce; halogen ion; precipitated gold solution; thermodynamics
Foundation item: Project(201407300993) supported by Xinjiang Uygur Autonomous Region Science and Technology
Received date: 2015-07-30; Accepted date: 2015-10-20
Corresponding author: ZHENG Ya-jie; Tel: +86-13974810738; E-mail: 13974810738@163.com
(编辑 王 超)
基金项目:新疆自治区科技支撑项目(201407300993)
收稿日期:2015-07-30;修订日期:2015-10-20
通信作者:郑雅杰,教授,博士;电话:13974810738;E-mail: 13974810738@163.com