盐湖副产硫酸钙转化法制备高纯氧化钙
冯雅丽1,马玉文1,李浩然2
(1. 北京科技大学 土木与环境工程学院,北京,100083;
2. 中国科学院过程工程研究所 生化工程国家重点实验室,北京,100190)
摘要:利用盐湖副产硫酸钙和碳酸氢铵溶液进行转化反应得到氧化钙的前驱体碳酸钙;然后,将其直接热解制备高纯氧化钙,分别考察物料配比、反应时间、反应温度等因素对硫酸钙转化率的影响,采用XRD和化学分析方法对产物进行分析,并对转化反应过程进行动力学分析。研究结果表明,在物料配比为1.3:1.0,反应时间为2.5 h,反应温度为50 ℃时, 硫酸钙的转化率可达到99.6%,将固相产物在1 000 ℃热解1 h后所制备的氧化钙纯度为99.7%。转化反应过程符合收缩核模型,其的数学表达式为kt=1-(1-X)1/3,反应的表观活化能为43.4 kJ/mol,该模型表明转化反应过程受界面化学反应控制。
关键词:盐湖硫酸钙;碳酸钙;高纯氧化钙;转化法;收缩核模型
中图分类号:TQ177.2 文献标志码:A 文章编号:1672-7207(2012)08-3308-06
Preparation of high-purity calcium oxide from salt lake by-product calcium sulfate by conversion method
FENG Ya-li1, MA Yu-wen1, LI Hao-ran2
(1. School of Civil and Environmental Engineering, University of Science and Technology Beijing, Beijing 100083, China;
2. Key State Laboratory of Biochemical Engineering, Institute of Process Engineering,
Chinese Academy of Sciences, Beijing 100190, China)
Abstract: The precursor of CaO was prepared by the conversion reaction between salt lake by-product CaSO4·2H2O and NH4HCO3 solution. And then high purity CaO was prepared by pyrolyzing. The effects of mole ratio of NH4HCO3 to CaSO4·2H2O, reaction time and temperature on the conversion rate were investigated respectively. The products were analyzed by XRD and chemical analytical method. The kinetic analysis of the conversion reaction process was made. The results show that the conversion rate of CaSO4·2H2O can reach 99.6% under the conditions of mole ratio 1.3:1.0, reaction time 2.5 h and temperature 50 ℃. The CaO with purity of 99.7% can be prepared by the pyrolysis of solid product at 1 000 ℃ for 1 h. The kinetic analysis demonstrates that the conversion process is in accordance with shrinking core model, which is expressed as a mathematical formula of kt=1-(1-X)1/3 and the apparent activation energy is 43.4 kJ/mol. The model indicates that the conversion process is controlled by the interface chemical reaction process.
Key words: salt lake calcium sulfate; calcium carbonate; high-purity calcium oxide; conversion method; shrinking core model
氧化钙是一种重要的无机材料,用于制造电石、液碱、漂白粉,广泛应用于建筑、道路、水产养殖、污水处理、冶金、水泥制造等多个行业,用作催化剂和有毒废水处理剂[1-2],还可以用作脱除煤气中的H2S和COS[3]。我国每年消耗氧化钙3 000多万t。氧化钙一般是通过热分解碳酸钙生产,而碳酸钙主要是由开山挖石提供,过度开采对山体植被和安全造成较大破坏。我国有丰富的盐湖钙资源[4],副产大量的硫酸钙,利用硫酸钙代替碳酸钙生产氧化钙既可以解决盐湖钙资源的利用问题,又可以减轻对生态环境的破坏,有利于发展循环经济。由于硫酸钙的热稳定性较强,采用直接热解或还原热解很难得到纯的氧化钙,梅林 等[5]研究了温度对硫酸钙分解率的影响,结果表明,硫酸钙在1 100 ℃以下基本不分解,在1 100~1 300 ℃之间开始缓慢分解,到1 350 ℃分解率仅为87.5%。Sohn等[6]研究了硫酸钙直接热分解过程中会产生二氧化硫,同时固相产物中会出现硫化钙。可见,硫酸钙直接热解存在反应温度高,而且所得固相产物中含有硫化钙等问题。张雪梅等[7]研究了硫酸钙还原热解的特性,分别对以H2,CH4,CO及C作还原剂时硫酸钙的分解反应及可能存在的副反应进行热力学计算,结果表明,还原热解硫酸钙虽然比直接热解温度低,但是,还原热解过程中有较多的副反应发生,会产生SO2,SO3,H2S和COS等有毒、有害气体。Song等[8]研究了硫酸钙与甲烷的反应,结果表明,反应产物中会有硫化钙的产生,同时气相产物中有CO和SO2。郑敏等[9]研究了CO与硫酸钙在不同温度下的反应行为,结果表明温度对硫酸钙还原反应历程和速率有显著影响,温度低于900 ℃,发生单一反应,固相产物为CaS,高于950 ℃会出现氧化钙,同时气相产物有羰基硫(COS)产生,有毒气体羰基硫对环境污染严重。因此,硫酸钙在还原条件下热解,反应条件不易控制,容易产生硫化钙,影响氧化钙的品质。针对硫酸钙热解不易得到较纯的氧化钙且会产生有毒、有害气体等问题,本文作者采用盐湖副产硫酸钙转化法制备氧化钙,即将硫酸钙和碳酸氢铵溶液在一定条件下进行固-液反应,从而避免了硫酸钙直接或还原热解反应中产生的有毒、有害气体,通过控制反应条件得到纯度较高的前躯体碳酸钙,然后将其热解得到高纯氧化钙,并对产物和反应过程进行分析。
1 实验
1.1 实验原理
硫酸钙转化法制备氧化钙的基本原理就是将硫酸钙与碳酸氢铵溶液在碱性条件下反应制备出碳酸钙,然后将碳酸钙热解得到氧化钙,反应方程式为:
CaSO4·2H2O+NH4HCO3+NH3·H2O=
(NH4)2SO4+CaCO3+3H2O (1)
CaCO3=CaO+CO2↑ (2)
根据硫酸钙和碳酸钙的溶解度的差异,碳酸钙和硫酸钙在水中(25 ℃)的溶度积(k)分别为2.8×10-9和9.1×10-6[10],碳酸钙的溶解度比硫酸钙小的多。因此,在一定条件下,硫酸钙可以向生成碳酸钙的方向转化,加入氨水可以保持反应体系呈碱性,保持足够量的碳酸根离子,使硫酸钙较完全地转化为碳酸钙[11]。
1.2 实验材料及试剂
所用硫酸钙为盐湖副产硫酸钙经提纯之后所得,将其磨细至粒度为75 μm备用;碳酸氢铵,分析纯;氨水,分析纯;蒸馏水。
1.3 实验仪器
实验仪器有:500 mL锥形瓶、AR1140电子天平、101-4A型电热恒温鼓风干燥箱、箱式电阻炉、DF-101S集热式恒温加热磁力搅拌器、SHZ-D(Ⅲ)循环水式真空泵、抽滤瓶和DZF-6020型真空干燥箱。
1.4 实验方法
先将一定量的碳酸氢铵加入到500 mL锥形瓶中,用蒸馏水溶解,液固比为10:1,然后按照不同摩尔比加入硫酸钙,同时加入氨水,使反应体系呈碱性,设定温度,在转速为400 r/min下进行磁力搅拌转化反应一定时间之后,趁热将反应产物转移至抽滤漏斗中进行真空抽滤,抽滤完之后用热蒸馏水对固相产物进行洗涤,固液分离之后,将得到的固相产物在电热恒温鼓风干燥箱中进行干燥,然后,在马弗炉中进行热解,得到固相产物;将所得液相蒸发浓缩至过饱和,冷却结晶、过滤,在真空干燥箱中进行干燥。
采用X-ray衍射仪(日本理学公司D/max-γB型:Cu靶,波长λ为0.154 06 nm,管电压为40 kV,管电流为100 mA,衍射速度为4 (°)/min,扫描范围2θ=10°~ 100°),根据HG/T 2226—2000《工业沉淀碳酸钙》[12]检测转化反应固相产物中碳酸钙的含量,采用EDTA滴定法[13]检测热解之后产物中氧化钙的含量。
将固液分离之后所得到的固相进行干燥,称质量,根据HG/T 2226—2000《工业沉淀碳酸钙》计算出碳酸钙的含量,根据反应式(1)得出硫酸钙转化率的计算公式为:
 (3)
(3)
式中,X为硫酸钙的转化率,%;m为硫酸钙的质量,g;m1为所得固相产物的质量,g;ω为固相产物中碳酸钙的质量分数,%。
2 结果与讨论
2.1 物料配比对硫酸钙转化率的影响
按照碳酸氢铵与硫酸钙的摩尔比(n(NH4HCO3)/n(CaSO4·2H2O))分别为1.0:1.0,1.1:1.0,1.2:1.0,1.3:1.0,1.4:1.0在温度为50 ℃下搅拌反应2.5 h,在不同物料配比下硫酸钙的转化率如图1所示。
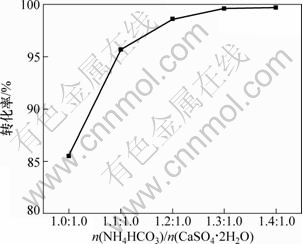
图1 物料配比对硫酸钙转化率的影响
Fig.1 Effect of mole ratio on conversion rate of CaSO4·2H2O
从图1可以看出:随着物料配比的增加,硫酸钙的转化率逐渐提高,说明有更多的硫酸钙转化为碳酸钙,当n(NH4HCO3)/n(CaSO4·2H2O)为1.3:1.0时,硫酸钙的转化率达到99.6%,硫酸钙转化为碳酸钙的程度趋于完全,说明保持碳酸氢铵过量有利于硫酸钙最大程度地转化,但物料比过大容易造成碳酸氢铵的浪费,而且不利于副产物硫酸铵的结晶。因此,确定合适的n(NH4HCO3)/n(CaSO4·2H2O)为1.3:1.0。
2.2 反应温度对硫酸钙转化率的影响
固定n(NH4HCO3)/n(CaSO4·2H2O)为1.3:1.0,分别在温度为20,30,40,50和60 ℃下搅拌反应2.5 h,在不同反应温度下,硫酸钙的转化率如图2所示。
从图2可以看出:硫酸钙的转化率随反应温度的升高而增大,在温度为50 ℃时,转化率达到最大值为99.6%;随后随着温度的升高转化率有下降趋势,当温度为60 ℃时,转化率降低为98.9%。这是因为温度从20 ℃升高到50 ℃时,反应温度升高加速了硫酸钙的溶解和扩散速率,促进了反应的进行,提高了硫酸钙的转化率;当温度大于50 ℃时,碳酸氢铵会出现部分分解的现象,使得反应体系中碳酸氢铵的浓度减少,降低了反应速率,导致硫酸钙的转化率下降。因此,确定合适的反应温度为50 ℃。
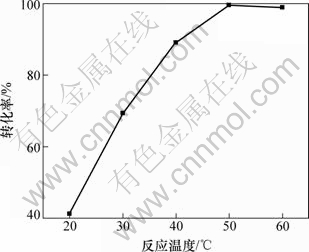
图2 反应温度对硫酸钙转化率的影响
Fig.2 Effect of reaction temperature on conversion rate of CaSO4·2H2O
2.3 反应时间对硫酸钙转化率的影响
固定n(NH4HCO3)/n(CaSO4·2H2O)为1.3:1,反应温度为50 ℃,设定反应时间分别为1.0,1.5,2.0,2.5和3.0 h进行反应。 在不同反应时间下硫酸钙的转化率如图3所示。
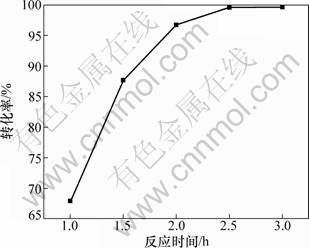
图3 反应时间对硫酸钙转化率的影响
Fig.3 Effect of reaction time on conversion rate of CaSO4·2H2O
从图3可以看出:随着反应时间的延长,硫酸钙的转化率逐渐增加,当反应进行了2.5 h时,硫酸钙的转化率达到99.6%,再延长时间,转化率没有增加。因此,确定合适的反应时间为2.5 h。
在最佳反应条件下进行转化反应,反应结束后将所得产物进行真空抽滤,固液分离之后,将所得固相产物进行烘干,置于马弗炉中在1 000 ℃热解1 h[14],得到氧化钙;所得液相进行蒸发浓缩至过饱和、冷却进行真空干燥得到硫酸铵晶体。
3 产物分析
采用X-ray衍射仪对所制备的产物进行分析,结果如图4和5所示。

图4 自制碳酸钙和分析纯碳酸钙的XRD图
Fig.4 XRD patterns of self-made CaCO3 and analytical pure CaCO3
从图4可以看出:自制碳酸钙的XRD图谱(图4(a))的衍射波峰尖锐,没有出现明显杂质峰,与分析纯碳酸钙的XRD图谱(图4(b))较一致。根据HG/T 2226—2000《工业沉淀碳酸钙》的检测方法,检测所得产物中碳酸钙的含量为99.5%,表明在最佳转化条件下,硫酸钙可以最大程度地转化为碳酸钙。
从图5(a)可以看出:沉淀物热解之后所得到氧化钙的XRD图谱没有明显杂质峰,与图5(b)中的分析纯XRD图谱较为一致,采用EDTA滴定法测定氧化钙含量为99.7%。表明所制备的氧化钙为高纯氧化钙。
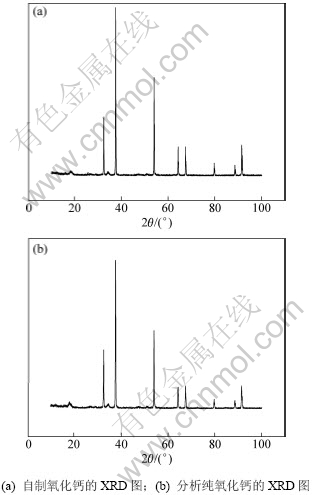
图5 自制氧化钙和分析纯氧化钙的XRD图
Fig.5 XRD patterns of self-made CaO and analytical pure CaO
4 转化反应过程动力学分析
由于硫酸钙是致密固体,反应时液体在固体内部的扩散阻力很大,不可能深入到固体反应物的内部,反应只能在液固接触面上进行,可以用收缩核模型来描述反应过程[15],
根据收缩核模型,当界面化学反应为控制步骤时,
kt=1-(1-X)1/3 (4)
当由液膜传质为控制步骤时,
kt=X (5)
当产物层内扩散为控制步骤时,
kt=1-3(1-X)2/3)+2(1-X) (6)
式中:k为反应速率常数;t为转化反应时间,h。
将实验结果分别代入动力学方程式(4)~(6)中,结果发现:只有由式(4)所得结果与实验结果相符,不同温度下转化反应的力学曲线如图6所示,对实验数据进行回归处理,得到不同温度下的反应速率常数。
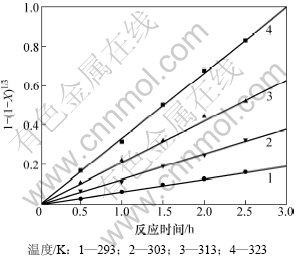
图6 不同温度下转化反应动力学曲线
Fig.6 Conversion reaction dynamic curves at different temperatures
由于速率常数k是温度的函数,温度T对k的影响可用Arrhenius公式[16]表示:
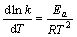 (7)
(7)
其积分形式为:
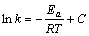 (8)
(8)
式中:Ea为表观活化能,kJ/mol;T为热力学温度,K;R为气体常数;C为与温度无关的积分常数。根据不同温度时的ln k 对1/T作图,结果如图7所示。
由图7可计算出表观活化能Ea=43.4 kJ/mol,可以进一步说明硫酸钙的转化过程受界面化学反应控制,即硫酸钙的转化率由最慢的界面化学反应速率决定。
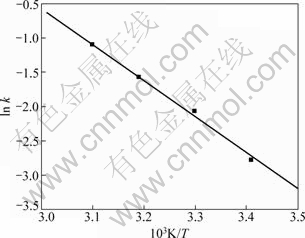
图7 lnk-1/T曲线
Fig.7 Curve of lnk-1/T
根据上述动力学研究可以推断硫酸钙转化过程中可分为如下5个步骤:
步骤1:液相中的碳酸氢铵由液体主流通过液膜传质到硫酸钙颗粒的外表面;
步骤2:液相中的碳酸氢铵由硫酸钙颗粒外表面,通过硫酸钙颗粒产物层扩散到未反应芯的界面;
步骤3:碳酸氢铵与硫酸钙颗粒进行化学反应;
步骤4:液体产物硫酸铵通过固体产物碳酸钙层由反应表面向外扩散;
步骤5:液体产物硫酸铵由颗粒外表面透过液膜扩散到液体主流。
其中,碳酸氢铵溶液与硫酸钙颗粒的反应速率是整个转化反应的控制速率,若要提高化学反应控制过程的速率,关键在于提高反应物碳酸氢铵的用量及反应温度。
5 结论
(1) 针对硫酸钙直接热解或还原热解制备氧化钙存在的问题,采用转化法制备出氧化钙,在n(NH4HCO3)/n(CaSO4·2H2O)为1.3:1,反应温度为50 ℃,反应时间为2.5 h时,硫酸钙的转化率为99.6%,硫酸钙可以最大程度地转化为碳酸钙,所得碳酸钙纯度为99.5%,将其在1 000 ℃下热解1 h,制备的氧化钙纯度为99.7%,说明硫酸钙转化法能够制备出高纯氧化钙。
(2) 动力学分析表明硫酸钙与碳酸氢铵溶液的转化反应属于有生成固体产物层的收缩核模型:kt=1-(1-X)1/3,其表观活化能为43.4 kJ/mol,转化反应过程由界面化学反应控制。
(3) 利用盐湖副产硫酸钙转化法制备高纯氧化钙,既充分利用了盐湖丰富的钙资源优势,又避免了硫酸钙直接热解或还原热解制氧化钙过程中有毒、有害气体的产生。该转化方法反应条件温和,易于控制,能耗低,同时,可以得到副产品硫酸铵,降低生产 成本。
参考文献:
[1] Tang Z X, Claveau D, Corcuff R, et al. Preparation of nano-CaO using thermal-decompositon method[J]. Materials Letters, 2008, 62(14): 2096-2098.
[2] Patel G, Pal U, Menon S. Removal of fluoride from aqueous solution by CaO nanoparticles[J]. Separation Science and Technology, 2009, 44(12): 2806-2826.
[3] 赵凤云, 胡永琪, 杜红霞, 等. 碳酸钙和氧化钙脱除煤气中H2S和COS的研究[J]. 化学世界, 2006(4): 208-211.
ZHAO Feng-yun, HU Yong-qi, DU Hong-xia, et al. Removal of H2S and COS in coal-gas with CaCO3 and CaO[J]. Chemical World, 2006(4): 208-211.
[4] 郑绵平, 卜令忠. 盐湖资源的合理开发与综合利用[J]. 矿产保护与利用, 2009(1): 17-22.
ZHENG Mian-ping, BU Ling-zhong. Rational development and comprehensive utilization of salt lake resources[J]. Conservation and Utilization of Mineral Resources, 2009(1): 17-22.
[5] 梅林, 李金洪, 王建春. 氧化物及复合添加剂对硫酸钙高温稳定性的影响[J]. 岩石矿物学杂志, 2005, 24(6):587-590.
MEI Lin, LI Jin-hong, WANG Jian-chun. The influence of oxides and compound additives on the stability of CaSO4[J]. Acta Petrologica et Mineralogical, 2005, 24(6): 587-590.
[6] Sohn H Y, Kim B S. A novel cyclic reaction system involving CaS and CaSO4 for converting sulfur dioxide to elemental sulfur without generating secondary pollutants: 1: Determination of process feasibility[J]. Industrial and Engineering Chemical Research, 2002, 41: 3081-3086.
[7] 张雪梅, 徐仁伟, 孙淑英, 等. 硫酸钙的还原热分解特性[J]. 环境科学与技术, 2010, 33(12F):144-148.
ZHANG Xue-mei, XU Ren-wei, SUN Shu-ying, et al. Study on the character of calcium sulfate reducing decomposition[J]. Environmental Science and Technology, 2010, 33(12F): 144-148.
[8] Song Q L, Xiao R, Deng Z Y, et al. Chemical-looping combustion of methane with CaSO4 oxygen carrier in a fixed bed reactor[J]. Energy Conversion and Management, 2008, 49: 3178-3187.
[9] 郑敏, 沈来宏, 肖军. 化学链燃烧钙基载氧体CaSO4与CO在不同温度下的反应行为[J]. 化工学报, 2008, 59(11): 2812-2818.
ZHENG Min, SHEN Lai-hong, XIAO Jun. Reduction of CaSO4 oxygen carrier with CO in chemical-looping combustion[J]. Journal of Chemical Industry and Engineering (China), 2008, 59(11): 2812-2818.
[10] 孙尔康, 张剑荣. 无机及分析化学实验[M]. 南京: 南京大学出版社, 2010: 50-76.
SUN Er-kang, ZHANG Jian-rong. Inorganic and analytical chemistry experiment[M]. Nanjing: Nanjing University Press, 2010: 50-76.
[11] 张茂林, 王永秋, 刘清理, 等. 磷石膏转化法制备硫酸铵的工艺研究[J]. 淮北煤师院学报, 2000, 21(2): 51-53.
ZHANG Mao-lin, WANG Yong-qiu, LIU Qing-li, et al. Technology in producing ammonium sulphate by way of transform with phosphogypsum[J]. Journal of Huaibei Coal Industry Teachers College. 2000, 21(2): 51-53.
[12] HG/T 2226—2000, 工业沉淀碳酸钙标准[S].
HG/T 2226—2000, Industry standard of calcium carbonate[S].
[13] 汪秋蕙. EDTA滴定法测定生石灰中的氧化钙[J]. 浙江冶金, 2005(3):31-32.
WANG Qiu-hui. Determination of CaO content in calcined lime by titrimetry method[J]. Journal of Zhejiang Metallurgy, 2005(3): 31-32.
[14] 余兆南. 碳酸钙分解的试验研究[J]. 热能动力工程, 1997, 12(4): 278-280.
YU Zhao-nan. An experimental study on the decomposition of calcium carbonate[J]. Journal of Engineering for Thermal Energy and Power, 1997, 12(4): 278-280.
[15] 姜志新. 湿法冶金分离工程[M]. 北京: 原子能出版社, 1993: 18-28.
JIANG Zhi-xin. Separation engineering of hydrometallurgy[M]. Beijing: Atomic Energy Press, 1993: 18-28.
[16] 韩其勇. 冶金过程动力学[M].北京: 冶金工业出版社, 1983: 29-32.
HAN Qi-yong. Kinetics of metallurgical process[M]. Beijing: Metallurgical Industry Process, 1983: 29-32.
(编辑 赵俊)
收稿日期:2011-09-19;修回日期:2011-12-29
基金项目:国家自然科学基金资助项目(20876160);国家高技术研究发展计划(“863”计划)项目(2007AA05Z158)
通信作者:冯雅丽(1967-),女,北京人,博士,教授,从事盐湖资源研究;电话:010-62311181;E-mail:ylfeng126@126.com