N’, N’’-双(5-烷基-1, 3, 4-噻二唑-2-)基
对苯二甲酰基双硫脲的合成与生物活性
王微宏,钟 宏,张文灵
(中南大学 化学化工学院,湖南 长沙,410083)
摘 要:为寻找高活性的含杂环农药,由中间体5-烷基-2-氨基-1, 3, 4-噻二唑与对苯二甲酰基二异硫氰酸酯反应,合成6种新的含1, 3, 4-噻二唑环的对苯二甲酰基双硫脲化合物Ⅲa-f。通过元素分析、红外光谱、核磁共振氢谱对所合成的化合物进行结构表征。生物活性测试结果表明:所合成的目标化合物对受试菌种小麦赤霉病菌,水稻纹枯病菌、棉枯萎病菌和油菜菌核病菌均表现出一定的抑制活性,但对棉枯萎病菌的抑制活性均较差。其中化合物Ⅲa和Ⅲb分别对油菜菌核病菌和水稻纹枯病菌有较强的抑制活性,抑制率分别为40.4%和51.6%,而Ⅲf的抑菌活性较弱。
关键词:1, 3, 4-噻二唑;硫脲;生物活性;合成
中图分类号:O621 文献标识码:A 文章编号:1672-7207(2009)01-0067-05
Synthesis and bioactivities of N’, N’’-bis(5-alkyl-1, 3, 4- thiodiazole-2-)yl terephthaloyl bisthioureas
WANG Wei-hong, ZHONG Hong, ZHANG Wen-ling
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: In order to find high active pesticide containing heterocycle, six novel terephthaloyl bisthioureas(Ⅲa-f) containing 1, 3, 4-thiodiazole were synthesized by the reaction of 2-amino-5-alkyl-1, 3, 4-thiodiazoles with terephthaloyl diisothiocyanate. Their structures were confirmed by elemental analysis, IR spectra and 1HNMR. The preliminary biological activity tests show that the title compounds have some fungicidal activities using Gibberella zeae, Rhizoctonia solani, Fusarium oxysporium and Sclerotinia sclerotiorum as the target fungus, but all of them have poor fungicidal activity to Fusarium oxysporium. The compounds Ⅲa and Ⅲb have good fungicidal activity to Sclerotinia sclerotiorum and Rhizoctonia solani, and their inhibition ratios are 40.4% and 51.6%, respectively.
Key words: 1, 3, 4-thiodiazole; thiourea; bioactivity; synthesis
芳酰基硫脲衍生物具有杀虫、抑菌、抗病毒、除草及调节植物生长等功效[1-5];含1, 3, 4-噻二唑环类化合物能除杂草、调节植物生长、杀菌、消炎、抗肿瘤等[6-7],因此,对这些化合物的研究正日益受到人们的重视。近年来为了寻找高活性的化合物,人们将1, 3, 4-噻二唑环嫁接到硫脲上,合成了具有抗结核活性[8]、抗菌活性[9]、植物生长调节活性[10]等含1, 3, 4-噻二唑环的硫脲衍生物。而含1, 3, 4-噻二唑环的双硫脲衍生物的合成未见报道。在此,本文作者运用分子设计,通过中间体5-烷基-2-氨基-1, 3, 4-噻二唑与对苯二甲酰基二异硫氰酸酯的反 应,获得含1, 3, 4-噻二唑环和芳酰基的双硫脲类化合物,运用元素分析、红外光谱(IR)、核磁共振氢谱(1HNMR)对其进行结构表征,同时还进行了的生物活性测试。
1 实 验
1.1 仪器与试剂
仪器为:美国Nicolet公司生产的G510P型红外光谱仪;美国PE公司产240C元素分析仪;瑞士Bruker公司生产的AV400核磁共振波谱仪;北京泰克仪器厂生产的X-6数字显微熔点测定仪,温度计未校正。
所有试剂均为分析纯。
1.2 5-烷基-2-氨基-1, 3, 4-噻二唑(Ⅰa-f)的合成
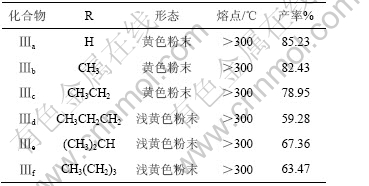
将10 g(0.11 mol)氨基硫脲加入100 mL的圆底烧瓶中,再加入有机羧酸0.11 mol和28.4 mL(0.33 mol)质量分数为36%的浓盐酸,磁力搅拌,回流3 h,自然冷却到室温,然后,用质量分数为40%氢氧化钠水溶液中和至pH 8~9[11],即产生大量的沉淀,冷却15 min,抽滤,洗涤干燥得粗产品,再用水重结晶,烘干得纯品5-烷基-2-氨基-1, 3, 4-噻二唑(Ⅰa-f)。化合物Ⅰa-f的形态、熔点和产率见表1。
表1 化合物Ia-f的形态、熔点和产率
Table 1 Appearance, melting point and yield of compounds Ia-f

1.3 对苯二甲酰基二异硫氰酸酯(Ⅱ)的合成
在装有搅拌器的250 mL圆底三颈烧瓶加入12.15 g(0.125 mol)硫氰酸钠和50 mL的二氯甲烷,将三颈烧瓶置于20 ℃低温恒温器中,在搅拌下向烧瓶中滴加溶有1.6 g催化剂PEG-400和10.15 g对苯二甲酰氯的二氯甲烷溶液(溶剂50 mL),约30 min加完。持续搅拌,保持20 ℃,反应3~4 h后结束[12]。滤去固体盐,用冰水洗涤并分离出有机相,即得对苯二甲酰基二异硫氰酸酯(Ⅱ)的二氯甲烷溶液,直接用于下一步反应。
1.4 N’, N”-双(5-烷基-1, 3, 4-噻二唑-2-)基对苯二甲酰基双硫脲(Ⅲa-f)的合成
将6.5 mmol的5-烷基-2-氨基-1, 3, 4-噻二唑加入50 mL的圆底烧瓶中,用THF溶解,待完全溶解后(约10 mL),加入2.6 mmol对苯二甲酰基二异硫氰酸酯(5-烷基-2-氨基-1, 3, 4-噻二唑与对苯二甲酰基二异硫氰酸酯的物质的量之比为2.5?1),混匀后,水浴加热,回流数小时,冷却至室温后,抽滤,洗涤、干燥得粗产品,经DMSO重结晶得纯品,化合物Ⅲa-f的形态、熔点和产率见表2。
表2 化合物Ⅲa-f的形态、熔点和产率
Table 2 Appearance, melting point and yield of compounds Ⅲa-f
化合物Ⅲa-f的合成路线如图1所示。
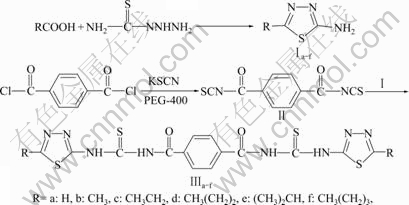
图1 N’, N’’-双(5-烷基-1, 3, 4-噻二唑-2-)基对苯二甲酰基双硫脲(Ⅲa-f)的合成
Fig.1 Synthesis of N’,N’’-bis(5-alkyl-1, 3, 4-thiodiazole-2-)yl terephthaloyl bisthiouareas(Ⅲa-f)
2 结果与讨论
2.1 元素分析
化合物Ⅲa-f的元素分析结果见表3。可见,目标化合物中各元素的含量与理论值基本吻合。
表3 化合物Ⅲa-f元素分析
Table 3 Elemental analyses of compounds Ⅲa-f

2.2 IR分析
用KBr压片法在400~4 000 cm-1范围内测定化合物Ⅲa-f的红外光谱,结果如表4所示。
表4 化合物Ⅲa-f的IR光谱数据
Table 4 Infrared spectral data of compounds Ⅲa-f
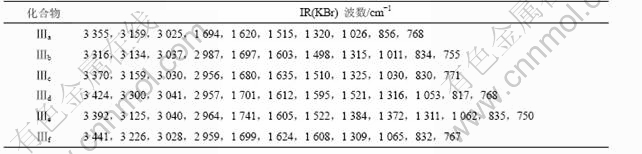
在波数为3 300~3 450 cm-1处的吸收强峰,对应于硫脲的N—H的伸缩振动;波数为3 020~3 050 cm-1处的吸收峰是苯环上的C—H伸缩振动所致;在2 950~2 990 cm-1处的吸收峰为饱和的C—H伸缩振动造成的;在1 680~1 750 cm-1处的强吸收峰对应于C=O的伸缩振动;在1 600~1 625 cm-1处的吸收峰为芳环上的碳架伸缩振动吸收峰。文献[13-14]认为硫代酰胺基在1 520~ 750 cm-1表现出4个特征的硫代酰胺谱带。由表3可看出,化合物Ⅲa-f明显的4个谱带为:C—N及NH振动组合产生的第1硫代酰胺谱带出现在1 515 cm-1附近,C—N及C=S贡献的第2硫代酰胺谱带出现在1 310~1 325 cm-1处,C—N和CNH 组合产生的第3硫代酰胺谱带出现在1 020~1 065 cm-1处,主要由C=S振动产生的第4硫代酰胺谱带出现在810~856 cm-1。在750~830 cm-1处的吸收峰对应于苯环上的2个相邻的氢的面外弯曲振动,表示苯环为对位二取代物。化合物Ⅲe在1 384 cm-1和1 372 cm-1处的吸收峰表明异丙基存在。
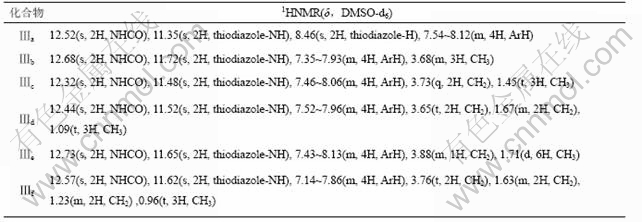
2.3 1HNMR分析
化合物Ⅲa-f的1HNMR光谱数据如表5如示。表5表明,各化合物的主要基团质子的化学位移与预期的化学位移基本一致。
表5 化合物Ⅲa-f的1HNMR光谱数据
Table 5 1HNMR spectrum data of compounds Ⅲa-f
2.4 生物活性测试
以小麦赤霉病菌Gibberella zeae,水稻纹枯病菌Rhizoctonia solani,棉枯萎病菌Fusarium oxysporium,油菜菌核病菌Sclerotinia sclerotiorum为受试菌种,采用琼脂扩散法进行生测试验。培养基为PDA(马铃薯、葡萄糖和琼脂),菌丝长好后用直径为0.4 cm的制块器制成菌块。测定时样品质量浓度为50 mg/L,每处理重复3次,取平均值。化合物Ⅲa-f的抑菌活性见表6。抑菌率η按下式计算:

由表6可知,化合物Ⅲa-f对受试菌种均表现出一定的抑制活性,但对棉枯萎病菌的抑制活性均表现较差。其中,Ⅲa和Ⅲb分别对油菜菌核病菌和水稻纹枯病菌有较强的抑制活性;Ⅲf的抑菌活性最弱。
表6 化合物Ⅲa-f的抑菌活性
Table 6 Fungicidal activities of compounds Ⅲa-f

3 结 论
a. 由氨基硫脲与羧酸在浓盐酸催化作用下,环合得到5-烷基-2-氨基-1, 3, 4-噻二唑。由对苯二甲酰氯与硫氰酸钾在相转移催化剂PEG-400催化作用下,发生亲核取代反应和异构化得对苯二甲酰基二异硫氰酸酯中间体。再由5-烷基-2-氨基-1, 3, 4-噻二唑与对苯二甲酰基二异硫氰酸酯经亲核加成反应合成目标产物。经元素分析、红外光谱、核磁共振氢谱等分析测试,证明所合成的化合物为标题化合物。
b. 所合成的目标化合物Ⅲa-f对受试菌种小麦赤霉病菌Gibberella zeae,水稻纹枯病菌Rhizoctonia solani,棉枯萎病菌Fusarium oxysporium以及油菜菌核病菌Sclerotinia sclerotiorum均表现出一定的抑制活性,但对棉枯萎病菌的抑制活性均较弱。其中,化合物Ⅲa和Ⅲb分别对油菜菌核病菌和水稻纹枯病菌有较强的抑制活性,抑制率分别为40.4%和51.6%,而Ⅲf的抑菌活性最弱。可见,1, 3, 4-噻二唑环上的R基团的结构对目标化合物的抑菌活性有较大的影响。
参考文献:
[1] 李英俊, 张治广, 靳 焜, 等. 酰基硫脲衍生物的合成、结构表征及生物活性研究[J]. 化学学报, 2007, 65(9): 834-840.
LI Ying-jun, ZHANG Zhi-guang, JIN Kun, et al. Synthesis, characterization and biological activities of acylthiourea derivatives[J]. Acta Chimica Sinica, 2007, 65(9): 834-840.
[2] 潘春跃, 蒋呈奎, 唐新村, 等. 查尔酮缩氨基硫脲类希夫碱的合成与表征[J]. 中南大学学报: 自然科学版, 2007, 38(1): 93-97.
PAN Chuen-yue, JIANG Cheng-kui, TANG Xin-chun, et al. Synthesis and characterization of schiff bases derived from chalcone and thiosemicarbazide[J]. Journal of Central South University: Science and Technology, 2007, 38(1): 93-97.
[3] SONG Bao-an, ZHANG Hua, JIN Lin-hong, et al. Synthesis and biological activity of a new type of thiourea derivatives[J]. Huaxue Tongbao, 2003(3): 200-202.
[4] Yonova P A, Stoilkova G M. Synthesis and biological activity of urea and thiourea derivatives from 2-aminoheterocyclic compounds[J]. J Plant Growth Regul, 2005, 23: 280-291.
[5] YANG Gui-chun, CHEN Zu-xing, ZHANG Zhao-jun. Combinatorial synthesis of novel thiourea derivatives on a modified poly(ethylene glycol)[J]. Reactive and Functional Polymers, 2002, 51(1): 1-6.
[6] 徐彦军, 王庆海, 武菊英, 等. 吡啶衍生物研究(X): 2-仲丁氨基-5-(2-芳氧吡啶-4-基)-1, 3, 4-噻二唑的合成及除草活性[J]. 农药学学报, 2007, 9(2): 189-192.
XU Yan-jun, WANG Qing-hai, WU Ju-ying, et al. Studies on pyridine derivatives(X): Synthesis and herbicidal activity of 2-sec-butylamino-5-(2-aryloylpyrid-4-yl)-1, 3, 4-thiodiazoles[J]. Chinese Journal of Pesticide Science, 2007, 9(2): 189-192.
[7] 胡国强, 孙茂峰, 谢松强, 等. 3-(4-哌嗪-1-苯基)-6-取代-s-三唑并[3,4-b][1, 3, 4]噻二唑盐酸盐的合成及抗菌活性[J]. 药学学报, 2007, 42(1): 54-57.
HU Guo-qiang, SUN Mao-feng, XIE Song-qiang, et al. Synthesis and antibacterial activity of 3-(4-piperazin-1-yl- phenyl)-s-triazolo[3,4-b][1, 3, 4] thiadiazole hydrochlorides[J]. Acta Pharmaceutica Sinica, 2007, 42(1): 54-57.
[8] Karakus S, Rollas S. Synthesis and antituberculosis activity of new N-phenyl-N’-[4-(5-alkyl/arylamino-1, 3, 4-thiadizole-2-yl) phenyl]thioureas[J]. Il Farmaco, 2002, 57(7): 577-581.
[9] 董兴高, 颜 玲, 宋新建, 等. N-[5-(3-吡啶基)-1, 3, 4-噻二唑-2-基]-N′-芳甲酰基脲的合成及抗菌活性[J]. 药学学报, 2007, 42(1): 108-110.
DONG Xing-gao, YAN Ling, SONG Xin-jian, et al. Synthesis and antimicrobial activity of N-[5-(3-pyridyl)-1, 3, 4-thiadiazol- 2-yl]-N′-aroyl urea[J]. Acta Pharmaceutica Sinica, 2007, 42(1): 108-110.
[10] 陈传兵, 杜 敏, 汪焱钢. N-(5-三氟甲基-1, 3, 4-噻二唑-2-基)-N'-芳氧乙酰基硫脲的合成及其生物活性[J]. 华中师范大学学报: 自然科学版, 2007, 41(2): 223-225.
CHEN Chuan-bing, DU Min, WANG Yan-gang. Synthesis and biological activity of N-(5-trifluoromethyl-1, 3, 4-thiodiazol- 2-yl)-N'-aryloxyacetyl thioure[J]. Journal of Huazhong Normal University: Natural Science, 2007, 41(2): 223-225.
[11] 乐长高, 丁健桦, 杨思金. 5-烷基-2-氨基-1, 3, 4-噻二唑的合成及应用[J]. 化学世界, 2002, 43(7): 366-368.
LE Zhang-gao, DING Jian-hua, YANG Si-jin. Synthesis and application of 5-alkyl-2-amino-1, 3, 4-thiodiazole[J]. Chinese Journal of Chemical World, 2002, 43(7): 366-368.
[12] 王 帅, 钟 宏, 吴 浩, 等. 对苯二甲酰基二异硫氰酸酯的合成工艺研究[J]. 精细化工中间体, 2005, 35(5): 21-23.
WANG Shuai, ZHONG Hong, WU Hao, et al. Study on the synthetic process of 1, 4-benzenecarbonyl Diisothiocyanate[J]. Fine Chemical Intermediates, 2005, 35(5): 21-23.
[13] Rao C N R. Contribution to the infrared spectra of organosulphur compounds[J]. Can J Chem, 1964, 42: 36-42.
[14] 李 健, 杨智宽. 苯基硫脲接枝壳聚糖的合成及其对金属离子吸附性能的研究[J]. 合成化学, 2004, 12(3): 255-258.
LI Jian, YANG Zhi-kuan. Synthesis of phenylthiourea grafted N-benzylidene chitosan and its adsorption properties for heavy metal ions[J]. Chinese Journal of Synthetic Chemistry, 2004, 12(3): 255-258.
收稿日期:2008-03-10;修回日期:2008-05-08
基金项目:国家自然科学基金资助项目(20476105);中南大学研究生教育创新工程资助项目(75208);中南大学文理研究基金资助项目(0601045)
通信作者:钟 宏(1961-),男,浙江龙泉人,博士,教授,从事有机合成研究;电话:0731-8830603;E-mail: zhongh@mail.csu.edu.cn