文章编号:1004-0609(2017)-01-0171-07
非等温条件下氢气还原Fe2O3-NiO制备镍铁合金的反应动力学
张海培1, 2,李 博1, 2,丁志广1, 2,魏永刚1, 2,周世伟1, 2
(1. 昆明理工大学 省部共建复杂有色金属资源清洁利用国家重点实验室,昆明 650093;
2. 昆明理工大学 冶金与能源工程学院,昆明 650093)
摘 要:主要研究不同质量比的Fe2O3-NiO在氢气气氛下还原过程的非等温动力学。根据热分析动力学研究方法,结合样品的质量损失曲线,获得了样品在非等温还原过程中的动力学曲线,并确定Fe2O3-NiO体系在氢气气氛下还原过程的最佳机理函数(G(α)=[-ln(1-α)]4),过程受随机成核和随后生长机理控制。结果表明:当样品中Fe2O3-NiO质量比从1:2变化到2:1时,还原反应过程的活化能从249.821 kJ/mol增加至390.074 kJ/mol;随着体系中NiO含量增加,还原反应开始的温度逐渐降低,还原产物物相由铁纹石相(Fe,Ni)和镍纹石相(Fe,Ni)逐渐转变为镍铁合金相(FeNi3),产物微观颗粒尺寸变得不均匀。通过建立数学模型,验证了反应过程中反应分数的模型计算值与实验测量值具有良好的相关性。
关键词:还原动力学;Fe2O3-NiO;镍铁合金;机理函数
中图分类号:TF815 文献标志码:A
镍是一种银白色金属,具有良好的力学强度、可塑性和很高的化学稳定性。镍主要用于不锈钢、高温材料、电镀和化工等行业[1-2]。因此,镍在国民经济发展中具有极其重要的地位。全球约2/3的镍用于生产不锈钢,镍原料的成本占奥氏体不锈钢生产成本的70%左右。我国的镍产品种类单一,主要为电解镍、氧化镍和镍铁。电解镍的价格极其昂贵,削弱了其在不锈钢产品市场的竞争力,在国外将镍铁作为冶炼不锈钢、合金钢和合金铸铁的镍合金剂已经非常普遍,镍铁合金的使用可以减少金属镍的消耗[3-5]。
制备镍铁合金较为成熟的方法是采用火法工艺从红土镍矿中冶炼镍铁。该方法是在高温条件下,以碳或硅作还原剂,对红土镍矿中的NiO、Fe2O3及其它氧化物进行还原,从而制得镍铁[6-8]。火法冶炼镍铁的方法又分鼓风炉熔炼法和回转窑—电炉还原熔炼法。鼓风炉熔炼法具有设备简单,基建投资低,硫化矿、氧化矿都能处理等优点,但随着冶炼厂对含镍原料要求的提高以及环境保护的需要,鼓风炉冶炼逐步被淘汰。回转窑-电炉还原熔炼法可处理含难熔物较多的原料,产出的镍铁品位高,显著地降低了电能和还原剂的消耗[9-11]。
BAHGAT等[12]研究了WO3-NiO-Fe2O3的还原和W-Ni-Fe三元合金的生成,通过氢气在800~1000 ℃还原WO3-NiO-Fe2O3混合物得到的质量分数为90%W-7%Ni-3%Fe合金的纳米晶体。ABDEL-HALIM等[13]研究了800~1100 ℃下固体碳还原Fe2O3-NiO体系生成Fe-Ni合金的动力学过程,发现铁氧化物的反应速率随体系温度和NiO含量的提高而增大,在低温下还原速率由布多尔反应控制。SARKISYAN[14]研究了等温条件下用碳和H2还原Fe2O3-NiO体系的动力学,发现碳做添加剂的Fe2O3-NiO混合物的还原过程,每个反应独立进行,无明显的交叉反应。CORES等[15]研究了用煤粉和氢气还原NiO-Fe3O4的机理,发现还原分两步进行,第一步是NiO和NiFe2O4的还原,第二步是铁氧化物的还原。李博等[16]采用热分析法研究了473~1223 ℃下煤粉还原红土镍矿动力学机理,发现还原过程分为473~773 ℃和773~1223 ℃两个阶段,反应活化能分别为171.91 kJ/mol和52.75 kJ/mol,反应速率由布多尔反应控制。
本文作者研究了不同质量配比的Fe2O3-NiO在氢气气氛中的非等温还原动力学。通过热重、XRD、扫描电镜等分析技术对样品的还原过程和产物进行分析,根据不同质量配比样品的质量损失曲线,建立动力学模型,获得动力学参数,为镍铁合金的生产提供了理论依据。
1 实验
本实验采用热天平分析法[17]进行实验研究,实验设备如图1所示,Fe2O3与NiO按照2:1、1:1、1:2的质量比进行配样,每组样品总质量5g。在纯度为99.95%的氢气气氛中,流量为100 mL/min,升温速率为10 ℃/min的条件下,从50 ℃升温至1000 ℃对样品进行还原焙烧,并分别用XRD、SEM表征其产物物相与微观结构变化。
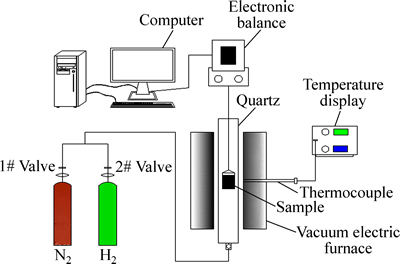
图1 实验装置示意图
Fig. 1 Schematic diagram of experimental equipment
2 结果与分析
2.1 氢气气氛下Fe2O3-NiO的还原过程的热重分析
图2所示为不同质量比样品的质量损失曲线。从图2中可以观察到质量损失率随温度的增大而增大,在270~515 ℃温度区间内,质量损失率不断增大,Fe2O3-NiO质量比为1:2、1:1、2:1的样品,质量损失率分别为75.7%、74.3%、72.9%。还原反应后期质量损失曲线近似一条直线和实际质量损失率与理论质量损失率近似相等,说明还原反应比较完全,Fe2O3、NiO被氢气还原为金属铁、金属镍以及镍铁合金,同时反应产物H2O在高温下被蒸发。
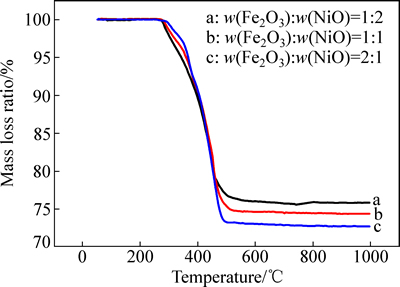
图2 在50~1000 ℃下氢气还原不同样品的质量损失曲线
Fig. 2 Mass loss curves of different samples reduced with hydrogen at temperatures of 50-1000 ℃

图3 在50~1000 ℃下氢气还原不同样品的反应分数曲线
Fig. 3 Reaction fractions of different samples reduced with hydrogen at temperatures of 50-1000 ℃
图3所示为氢气还原不同样品的反应分数曲线。从图3中可以看出,在反应的初期和末期反应分数基本保持不变。在反应进行的初始阶段,3个不同质量比的样品反应速率最高;随着反应不断进行,各样品反应速率逐渐减小,在反应的末期减小为零。随着样品中NiO含量的增加,反应温度也随之降低,可推断出在反应的初始阶段,NiO优先被还原为镍,而生成的金属镍又作为反应的催化剂,促进了反应的进行[18]。
图4所示为不同样品还原产物的XRD谱。从图4中可以看出,随着样品中NiO含量的不断增大,衍射峰强度也不断增强。3种不同样品主要物相为铁纹石(Fe,Ni)、镍纹石(Fe,Ni)、铁镍矿(FeNi3)。Fe2O3-NiO质量比为1:2的样品主要还原产物物相为铁镍矿(FeNi3),随着样品中NiO含量不断减少,谱线中逐渐可以观察到铁纹石(Fe,Ni)、镍纹石(Fe,Ni)。
利用扫描电镜(SEM)对不同样品的还原产物进行形貌分析。从图5可以观察出,3种不同样品的还原产物具有相似的内部构造,随着样品中NiO含量增加,产物微观颗粒尺寸变得不均匀,表面结构也随之变得越来越不规则。

图4 不同样品的XRD谱
Fig. 4 XRD patterns of different samples

图5 不同配比样品还原产物的SEM像
Fig. 5 SEM images of different reduced samples
2.2 Fe2O3-NiO的还原过程动力学机理分析
采用热分析动力学中Coats-Redfern法[19-20],得到非等温条件下动力学方程式(1)为:
 (1)
(1)
式中:α为反应分数;β为加热速率;A为指前因子;E为活化能;G(α)为机理函数;R为摩尔气体常量,8.314 J/(mol·K)。对于一般的反应温度区间和大部分E而言,E/(RT)≥1,因此1-2(RT)/E≈1。以ln(G(α))/T2对1/T作图,得到一条直线,斜率为-E/R,指前因子A由截距求出。
通过对气-固相反应动力学常用的20种机理函数的ln(G(α))/T2对1/T的动力学曲线分析,确定了相关系数最大的Avrami-Erofeev方程(n=4)为最佳机理函数,对应随机成核和随后生长机理[21-22],其积分形式G(α)=[-ln(1-α)]4,用Coats-Redfern法,得到该还原过程的动力学方程为
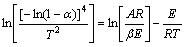 (2)
(2)
不同配比的样品的ln{[-ln(1-α)]4/T2}对1/T的关系曲线如图6所示,从图6中可以看出,ln{[-ln(1-α)]4/T2}对1/T的关系曲线都近似一条直线。得到的还原过程动力学曲线的相关系数R值均在0.99以上,说明回归效果均是高度显著和可信的。
用拟合得到的直线的斜率和截距分别求出不同配比样品还原过程的活化能和指前因子。不同配比样品的活化能和指前因子如表2所列。从表2中可以看出,活化能随着样品中NiO含量的增大而减小,活化能越高意味着反应越难进行,从而说明样品中NiO含量越高反应越容易进行,表明NiO在整个还原反应中作为催化剂,促进反应进行[23]。
表1 部分气固反应机理函数及相关系数
Table 1 Correlation coefficient of some mechanism function in gas-solid reaction

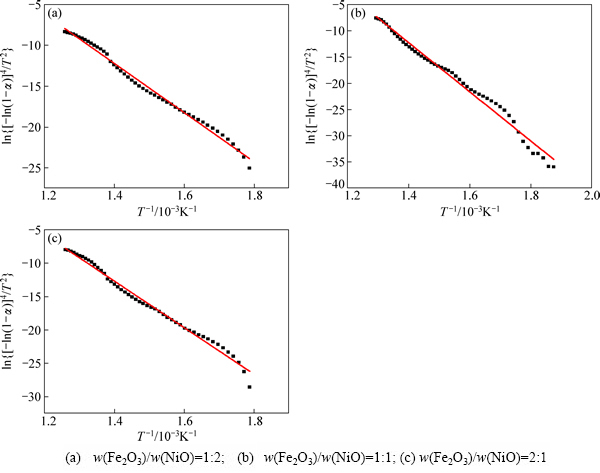
图6 不同样品的ln{[-ln(1-α)]4/T2}和1/T关系曲线
Fig. 6 Plots of ln{[-ln(1-α)]4/T2} versus 1/T for different samples
表2 不同配比样品的活化能和指前因子
Table 2 Apparent activation energies E and pre-exponential factors A at different proportions compacts reduction reactions

结合图2和表2发现,活化能随着质量损失增大而增大。可以通过随机成核和随后生长模型的物理意义解释,反应最初发生在某些局域的点上(晶格缺陷处),随后这些相邻近的分解产物聚集成一个新物相的核,然后核周围的分子继续在核上发生界面反应,旧物相不断消失,新物相不断生长和扩展[24]。在核形成后,由于新物相的不断生长和扩展需要能量,质量损失越大说明新物相生长和扩散的过程越长,需要的能量也就越多,这就可以解释活化能随质量损失增大而增大的现象[25]。
图7所示为不同样品还原过程反应分数的测量值与理论值。活化能和指前因子已由ln{[-ln(1-α)]4/T2}和1/T拟合出直线的截距和斜率求出,将活化能和指前因子的计算结果带入该还原过程的动力学方程式,建立数学模型[26]。将反应分数作为升温时间的函数,计算不同样品的还原反应分数,从图7可以看出,除了个别反应阶段稍有偏差外,其他的还原反应阶段的测量值与理论值有良好的相关性,个别阶段的偏差可能是由Ni的催化作用引起的。
3 结论
1) 样品中NiO含量越高,反应开始的时间越早,反应越容易进行,NiO在整个反应中优先被还原为Ni,而Ni作催化剂,促进了Fe2O3的还原。
2) 随着样品中NiO含量增大,还原产物中的铁镍矿(FeNi3)相越多,而铁纹石(Fe,Ni)相、镍纹石(Fe,Ni)相随之减少,产物颗粒尺寸变得不均匀。
3) 通过对20种机理函数的计算,选定了Avrami-Erofeev方程(n=4)为最佳机理函数,还原过程主要由随机成核和随后生长机理控制。
4) 对于不同质量比的Fe2O3-NiO样品,还原过程的活化能和指前因子随着样品中NiO含量的增加而减小,NiO起到促进反应进行、提高反应速率的作用。
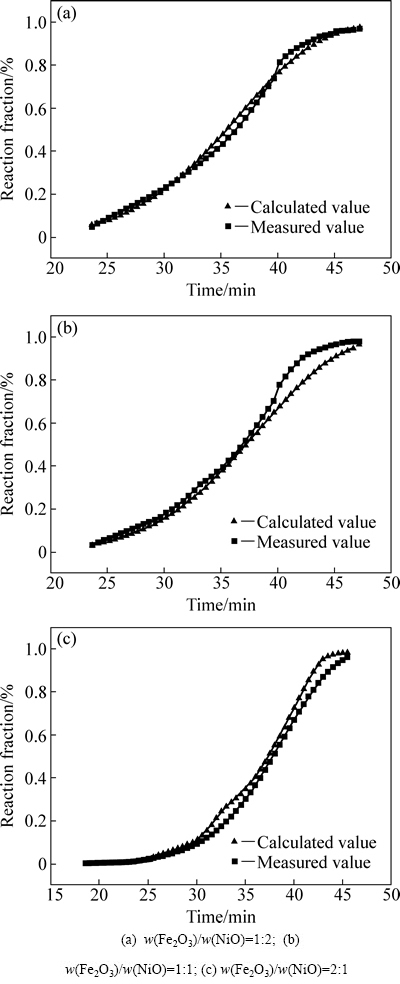
图7 不同样品反应分数的测量值与理论值
Fig. 7 Calculated values and measured values of reaction fraction for different samples
REFERENCES
[1] 彭容秋. 镍冶金[M]. 长沙: 中南大学出版社, 2005: 2-4, 165-166.
PENG Rong-qiu. Metallurgy of nickel[M]. Changsha: Central South University Press, 2005: 2-4, 165-166.
[2] 刘 岩, 翟玉春, 王 虹. 镍生产工艺研究进展[J]. 材料导报, 2006, 20(3): 79-81, 96.
LIU Yan, ZHAI Yu-chun, WANG Hong. Research on production process of nickel[J]. Materials Review, 2006, 20(3): 79-81, 96.
[3] 李志茂, 朱 彤, 吴家正. 镍资源的利用及镍铁产业的发展[J]. 中国有色冶金, 2009(1): 29-32.
LI Zhi-mao, ZHU Dan, WU Jia-zheng. Using of the nickel resource and development of the ferronickel industry[J]. China Nonferrous Metallurgy, 2009(1): 29-32.
[4] 王成彦, 尹 飞, 陈永强. 国内外红土镍矿处理技术及进展[J]. 中国有色金属学报, 2008, 18(S1): s1-s8.
WANG Cheng-yan, YIN Fei, CHEN Yong-qiang. Worldwide processing technologies and progress of nickel laterites[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(S1): s1-s8.
[5] LI Yan-jun, SUN Yong-sheng, HAN Yue-xin, GAO Peng. Coal-based reduction mechanism of low-grade laterite ore[J]. Transactions of Nonferrous Metals Society of China, 2013, 23(11): 3428-3433.
[6] 潘 成, 吕学伟, 白晨光, 郭恩光, 陈 攀, 刘 梅. 红土镍矿半熔融还原生产含镍珠铁[J]. 中南大学学报(自然科学版), 2014, 45(1): 16-24.
PAN Cheng,  Xue-wei, BAI Chen-guang, GUO En-guang, CHEN Pan, LIU Mei. Ferronickel nugget production from nickel laterite by semi-molten reduction[J]. Journal of Central South University (Science and Technology), 2014, 45(1): 16-24.
Xue-wei, BAI Chen-guang, GUO En-guang, CHEN Pan, LIU Mei. Ferronickel nugget production from nickel laterite by semi-molten reduction[J]. Journal of Central South University (Science and Technology), 2014, 45(1): 16-24.
[7] 郭亚光, 朱 荣, 吕 明, 郭明威, 王永威, 周春芳. 红土镍矿选择性还原-熔分制备镍铁合金[J]. 北京科技大学学报, 2014, 36(5): 584-591.
GUO Ya-guang, ZHU Rong,  Ming, GUO Ming-wei, WANG Yong-wei, ZHOU Chun-fang. Extraction of a nickel-iron alloy from nickel laterite ore through selective reduction and smelting process[J]. Journal of University of Science and Technology Beijing, 2014, 36(5): 584-591.
Ming, GUO Ming-wei, WANG Yong-wei, ZHOU Chun-fang. Extraction of a nickel-iron alloy from nickel laterite ore through selective reduction and smelting process[J]. Journal of University of Science and Technology Beijing, 2014, 36(5): 584-591.
[8] 李光辉, 饶明军, 姜 涛, 黄晴晴, 史唐明, 张元波. 红土镍矿还原焙烧-磁选制取镍铁合金原料的新工艺[J]. 中国有色金属学报, 2011, 21(12): 3137-3142.
LI Guang-hui, RAO Ming-jun, JIANG Tao, HUANG Qing-qing, SHI Tang-ming, ZHANG Yuan-bo. Innovative process for preparing ferronickel materials from laterite ore by reduction roasting-magnetic separation[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(12): 3137-3142.
[9] 刘志国, 孙体昌, 高恩霞, 王晓平. 蛇纹石矿物的高温相变对红土镍矿直接还原的影响[J]. 中国有色金属学报, 2015, 25(5): 1332-1338.
LIU Zhi-guo, SUN Ti-chang, GAO En-xia, WANG Xiao-ping. Effect of high-temperature phase transition of serpentine mineral on direct reduction roasting of laterite nickel ore[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(5): 1332-1338.
[10] GUO Qiang, QU Jing-kui, HAN Bing-bing, WEI Guang-ye, ZHANG Pei-yu, QI Tao. Dechromization and dealumination kinetics in process of Na2CO3-roasting pretreatment of laterite ores[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(5): 3979-3986.
[11] 刘志宏, 杨慧兰, 李启厚, 朱德庆, 马小波. 红土镍矿电炉熔炼提取镍铁合金的研究[J]. 有色金属(冶炼部分), 2010(2): 2-5.
LIU Zhi-hong, YANG Hui-lan, LI Qi-hou, ZHU De-qing, MA Xiao-bo. Study on the process of extraction ferronickel from laterite by electric smelting[J]. Nonferrous Metals (Extractive Metallurgy), 2010(2): 2-5.
[12] BAHGAT M, PAEK M K, PAK J J. Reduction investigation of WO3/NiO/Fe2O3 and synthesis of nanocrystalline ternary W-Ni-Fe alloy[J]. Journal of Alloys and Compounds, 2009, 472: 314-318.
[13] ABDEL-HALIM K S, KHEDR M H, NASR M I, ABDEL-WAHAB M S. Carbothermic reduction kinetics of nanocrystallite Fe2O3/NiO composites for the production of Fe/Ni alloy[J]. Journal of Alloys and Compounds, 2008, 463(1/2): 585-590.
[14] SARKISYAN L. Mechanism and kinetics of reduction of complex oxides of the NiO-Fe2O3 system[J]. Powder Metallurgy and Metal Ceramics, 1986, 25(10): 832-837.
[15] CORES A, FORMOSO A, LARREA M T, QRTIZ J. Kinetic regularities in joint reduction of nickel and iron oxides under non-isothermal conditions[J]. Iron making Steelmaking, 1989, 16(6): 446-449.
[16] 李 博, 魏永刚, 王 华. 红土镍矿的固相还原动力学[J], 过程工程学报, 2011, 11(5): 767-771.
LI Bo, WEI Yong-gang, WANG Hua. Solid state deoxidization kinetics of nickel laterite ore[J]. The Chinese Journal of Process Engineering, 2011, 11(5): 767-771.
[17] 肖天来, 冯小平, 谢峻林, 何 峰. 热天平的研制[J]. 武汉理工大学学报, 2001, 23(8): 24-26.
XIAO Tian-lai, FENG Xiao-ping, XIE Jun-lin, HE Feng. Flexibility curvature method in the damage detection of structure[J]. Journal of Wuhan University of Technology, 2001, 23(8): 24-26.
[18] LI Bo, WEI Yong-gang, WANG Hua. Non-isothermal reduction kinetics of Fe2O3-NiO composites for formation of Fe-Ni alloy using carbon monoxide[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(11): 3710-3715.
[19] 胡荣祖, 史启祯. 热分析动力学[M]. 第二版. 北京: 科学出版社, 2008: 2-8.
HU Rong-zu, SHI Qi-zhen. Thermal analysis kinetics[M]. 2nd edition. Beijing: Science Press, 2008: 2-8.
[20] 李 歌, 李增和, 马鸿文, 陈登利. 热重分析法研究氢氧化镁纳米粉体的非等温分解动力学[J]. 化工学报, 2014, 65(2): 576-582.
LI Ge, LI Zeng-he, MA Hong-wen, CHEN Deng-li. Non-isothermal decomposition kinetics of nano-Mg(OH) using thermal gravimetric analysis[J]. CIESC Journal, 2014, 65(2): 576-582.
[21] 赵留成, 孙春宝, 张舒婷, 谢文清, 郑新烟, 刘 柯. 主要载金硫化物黄铁矿的热分解动力学特性[J]. 中国有色金属学报, 2015, 25(8): 2212-2217.
ZHAO Liu-cheng, SUN Chun-bao, ZHANG Shu-ting, XIE Wen-qing, ZHENG Xin-yan, LIU Ke. Characteristic of thermal decomposition kinetics of main gold-bearing sulfides pyrite[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(8): 2212-2217.
[22] 李 辉, 张乐乐, 段永华, 闵永刚. 高二氧化碳浓度下石灰石的热分解反应动力学[J]. 硅酸盐学报, 2013, 41(5): 637-643.
LI Hui, ZHANG Le-le, DUAN Yong-hua, MIN Yong-gang. Kinetics of thermal decomposition reaction of limestone at high carbon dioxide concentration[J]. Journal of the Chinese Ceramic Society, 2013, 41(5): 637-643.
[23] ZHANG Y, WEI W, YANG X, WEI F. Reduction of Fe and Ni in Fe-Ni-O systems[J]. Journal of Mining and Metallurgy, Section B: Metallurgy, 2013, 49: 13-20.
[24] 日本化学学会. 无机固态反应[M]. 董万堂, 董绍俊, 译. 北京: 科学出版社, 1985: 64.
The Society of Chemical Engineers. Japan. Inorganic solid state reaction[M]. DONG Wan-tang, DONG Zhao-jun, translate. Beijing: Science Press, 1985: 64.
[25] 郑 瑛, 陈小华, 周英彪, 郑楚光. CaCO3分解机理和动力学参数的研究[J]. 华中科技大学学报(自然科学版), 2002(12): 86-88.
ZHENG Ying, CHEN Xiao-hua, ZHOU Ying-biao, ZHENG Chu-guang. The decomposition mechanism of CaCO3 and its kinetics parameters[J]. Journal of Huazhong University of Science and Technology (Nature Science), 2002(12): 86-88.
[26] 马兴亚, 姜茂发, 汪 琦, 王向辉. 铁矿-煤球团反应过程动力学及模型[J]. 东北大学学报(自然科学版), 2002, 23(5): 440-443.
MA Xing-ya, JIANG Mao-fa, WANG Qi, WANG Xiang-hui. Kinetics and model of reaction process of iron ore coal pellet[J]. Journal of Northeastern University (Natural Science), 2002, 23(5): 440-443.
Reduction kinetics of Fe2O3-NiO composites for production of Fe/Ni alloy using hydrogen under non-isothermal conditions
ZHANG Hai-pei1, 2, LI Bo1, 2, DING Zhi-guang1, 2, WEI Yong-gang1, 2, ZHOU Shi-wei1, 2
(1. State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization,
Kunming University of Science and Technology, Kunming 650093, China;
2. Faculty of Metallurgy and Energy Engineering,
Kunming University of Science and Technology, Kunming 650093, China)
Abstract: For the different mass ratio of Fe2O3 and NiO, the non-isothermal kinetics of reduction process using hydrogen as agent was investigated. According to the method of thermal analysis kinetics, combined with mass loss curves of sample, the deoxidization kinetics of the sample in reduction of non-isothermal process and the best mechanism function(G(α)=[-ln(1-α)]4) for reduction process of Fe2O3-NiO system in hydrogen atmosphere were obtained. It is found that the reduction is dominated by the random nucleation and then growth process. The results show that when the Fe2O3-NiO mass ratio is changed from 1:2 to 2:1, the activation energies of reduction process increase from 249.821 kJ/mol to 390.074 kJ/mol. With the content of NiO increasing,the initial temperature of reduction reaction reduces gradually, the Kamacite(Fe, Ni) and the Taenite(Fe, Ni) gradually convert into the awaruite (FeNi3). The particle-size of the product after reduction become, nonuniform. Based on the built mathematical model, the calculated values of mathematical model and the measured values of reaction fraction are fit in well.
Key words: reduction kinetics; Fe2O3-NiO; Fe-Ni alloy; mechanism function
Foundation item: Project (U1302274, 51304091) supported by the National Natural Science Foundation of China
Received date: 2015-11-20; Accepted date: 2016-03-30
Corresponding author: LI Bo; Tel: +86-15987127468; E-mail: libokmust@163.com
(编辑 何学锋)
基金项目:国家自然科学基金资助项目(U1302274,51304091)
收稿日期:2015-11-20;修订日期:2016-03-30
通信作者:李 博,副教授,博士;电话:15987127468;E-mail:libokmust@163.com