DOI:10.19476/j.ysxb.1004.0609.2018.09.20
独居石脱除工业硫酸锌溶液中氟的吸附性能
赖延清,杨 凯,郭伟昌,杨 超,田忠良,张 凯,李 劼
(中南大学 冶金与环境学院,长沙 410083)
摘 要:采用独居石对工业硫酸锌溶液进行吸附脱氟,研究吸附时间、吸附温度、吸附剂添加量、pH等工艺参数对脱氟效果的影响,分析其吸附动力学及等温线特征,初步探讨独居石的脱氟机理。结果表明:独居石中的铈与工业硫酸锌溶液中氟的相互作用使氟得以脱除;采用独居石吸附脱氟符合伪一级动力学模型和Langmuir吸附等温线模型,其最大理论吸附容量可达5.29 mg/g;当工业硫酸锌溶液在温度70 ℃、吸附时间65 min、添加量为25 g/L、pH值为1时,氟的脱除率可达到46.06%,具有良好的脱氟效果。
关键词:独居石;工业硫酸锌溶液;脱氟
文章编号:1004-0609(2018)-09-1893-07 中图分类号:TF813 文献标志码:A
世界上超过80%的锌是通过湿法炼锌技术生产 的[1-2],其主要工艺流程为焙烧→浸出→净化→电积[3-4]。但是,近年来随着矿产资源的过度开发,锌矿石的品位降低,锌冶炼企业开始提高对二次锌资源的利用[5-6]。这将导致电积过程硫酸锌溶液中氟离子浓度过高,从而破坏阴极铝板表面的氧化膜,使阴极铝板与析出的锌形成锌铝合金而发生粘接,导致剥板困难,增加工作量,同时使铝板大量消耗[7-8],加重设备的腐蚀,最终导致企业成本增加。
目前硫酸锌溶液脱氟研究的主要方法有化学沉淀法[9]、絮凝沉淀法[10]、萃取法[11-12]、离子交换法[13-14]等。但这些方法都存在不同的缺点,如脱氟率低,成本过高,工艺复杂等,无法在工业中真正得到应用。因此,寻找其他有效方法切实解决硫酸锌溶液氟含量过高的问题成为锌冶炼企业的迫切要求。吸附法[15]由于具有环境适应性好,成本低以及操作简单等优点,在高氟饮用水的处理中应用广泛[16],其中,采用含钙[17-18]、铝、镁[15]、稀土[16, 19]等相关的吸附剂脱氟的研究居多。吸附法也因其具有众多优点,被认为在硫酸锌溶液脱氟中具有巨大的潜力。此前有研究者用含钙铝吸附剂[20]和活性氧化铝[21]对硫酸锌模拟溶液进行脱氟研究,可以达到良好的效果,但针对工业硫酸锌溶液脱氟的研究鲜有报道。在各类吸附剂中,脱氟效果最好的是稀土类物质,但是稀土盐类或氧化物的价格比较昂贵,往往成本较高。因此,寻找富含稀土元素的天然物质脱氟将会是一种有效的方法。
独居石是一种富含稀土元素的天然矿物,其主要成分为镧和铈的磷酸盐,常被用来提取稀土,而稀土元素镧、铈对氟离子又具有很强的亲和性。因此,本文以富含稀土元素的独居石为吸附剂,研究工艺参数对独居石脱氟效果的影响;同时,分析了独居石脱氟的动力学和吸附等温线,在此基础上,获得了独居石脱氟的理论吸附容量;最后结合XPS分析对独居石的脱氟机理进行探讨。
1 实验
1.1 实验原料
本实验中所用独居石主要成分为(Ce、La)PO4,其中铈氧化物占39%~74%,镧稀土氧化物占0~5%,另外,还有少量的钙镁硅酸盐。实验中所用硫酸锌溶液为某锌冶炼厂的硫酸锌原液,其主要成分及浓度如下。
1.2 脱氟实验
将恒温水浴振荡器设定到指定温度t,待达到温度t后,称取质量为m的独居石,置于250 mL锥形瓶,然后量取100 mL工业硫酸锌溶液加入锥形瓶,用玻璃棒搅匀,置于恒温震荡器中,调节转速130 r/min,震荡一定时间后,静置。
表1 工业硫酸锌溶液的主要成分
Table 1 Main ingredients of industrial zinc sulfate solution

1.3 氟离子的检测
控制不同的工艺参数:吸附时间t、溶液温度t,吸附剂添加量m和溶液pH(用稀H2SO4调节)来进行脱氟实验。取吸附后溶液的上清液稀释100倍,用氟离子计(PXSJ-216,上海仪电科学仪器股份有限公司)测量后计算得出剩余氟离子浓度ρe(mg/L),通过式(1)和(2)分别计算独居石的脱氟率γ(%)和吸附容量qe(mg/g):
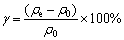 (1)
(1)
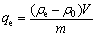 (2)
(2)
式中:ρ0为初始氟离子浓度,mg/L;V为溶液体积,L;m为独居石的加入量,g。本实验中溶液体积V为0.1 L,独居石加入量(m)为2.5 g。
1.4 XPS分析
采用X射线光电子能谱(XPS, ThermoFisherESCALAB250xi)对吸附氟前后的独居石进行表征,以通过观察脱氟前后不同元素所对应的光子能量值的变化,获得独居石的对工业硫酸锌溶液中氟的吸附机理。
2 结果与讨论
2.1 工艺参数对脱氟效果的影响
图1所示为吸附时间和温度对独居石脱氟效果的影响。由图1(a)可知,随着吸附时间的延长,独居石的脱氟率呈上升趋势。在前25 min脱氟率上升缓慢;25~45 min之间,脱氟率提升速度较快;45~65 min,脱氟率上升速度逐渐放缓;65 min时,脱氟率升至29.67%;65~85 min脱氟率基本保持不变,这说明吸附已经达到平衡。所以,选择65 min作为最佳吸附时间。由图1(b)可知,随着硫酸锌溶液温度的升高,独居石脱氟率逐渐升高,但升高的幅度逐渐变小。工业硫酸锌溶液温度从20 ℃升高至70 ℃,脱氟率从25.44%升高至33.41%;温度继续升高到80 ℃,脱氟率基本不变。因此,最佳吸附温度为70 ℃。

图1 吸附时间和温度对独居石脱氟效果的影响(ρ=25 g/L、pH=5.1)
Fig. 1 Effect of adsorption time (a) and temperature (b) on defluoridation efficiency by monazite (ρ=25 g/L, pH=5.1)
图2所示为独居石添加量对脱氟效果的影响。由图2可知,随着独居石添加量的增加,脱氟率逐渐上升,当添加量为25 g/L时,脱氟率达到33.46%;添加量继续增加时,脱氟率保持不变,所以独居石的添加量应控制在25 g/L。图3所示为pH对独居石脱氟效果的影响。由图3可知,在吸附时间、温度和添加量等参数确定的情况下,随着硫酸锌溶液pH的减小,独居石的脱氟率是逐渐升高的,即pH越低,独居石的脱氟效果越好。当溶液pH值从5.0降至1.0,脱氟率从33.26%升高至46.06%。所以,最佳溶液pH值为1。
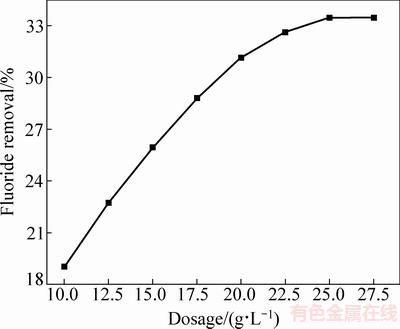
图2 添加量对独居石脱氟效果的影响(t=65 min、θ=70 ℃、pH=5.1)
Fig. 2 Effect of adsorbent dosage on defluoridation efficiency by monazite (t=65 min, θ=70 ℃, pH=5.1)

图3 pH对独居石脱氟效果的影响(t=65 min、θ=70 ℃、ρ=25 g/L)
Fig. 3 Effect of pH on defluoridation efficiency by monazite (t=65 min, θ=70 ℃, ρ=25 g/L)
2.2 动力学分析
描述吸附动力学要有伪一级和伪二级动力学两种模型。图4(a)和(b)所示分别为独居石脱氟的伪一级和伪二级动力学模型的拟合图。伪一级动力学模型的线性表达式为
 (3)
(3)
式中:qe和qt分别为吸附平衡和时间t时的吸附量(mg/g);k1为伪一级动力学模型的速率常数;qe和k1可以通过图4(a)的拟合曲线的截距和斜率分别计算 出来。
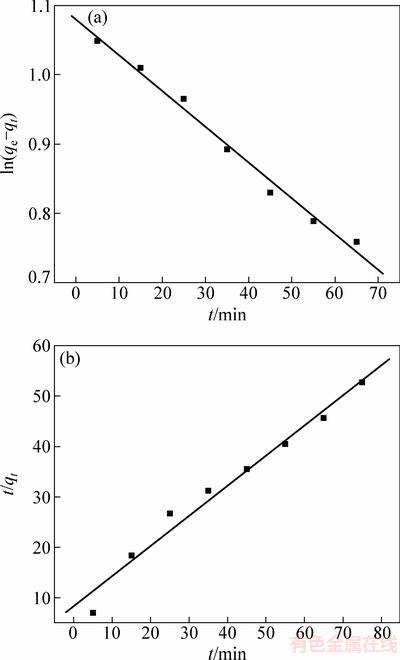
图4 独居石脱氟的伪一级和伪二级动力学模型
Fig. 4 Pseudo-first order (a) and pseudo-second order (b) kinetic models of fluoride removal on monazite
伪二级动力学模型的表达式为
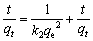 (4)
(4)
k2为伪一级动力学模型的速率常数,qe和k2可以通过图4(b)的拟合曲线的截距和斜率分别计算出来。
表2所列为独居石脱氟的动力学模型参数表,从表中可以得出,伪一级动力学模型拟合的相关系数0.9877,大于伪二级动力学模型的相关系数0.9750。而且其计算得出的吸附量2.94 mg/g与实验值3.56 mg/g更接近,因此独居石脱氟过程更符合伪一级动力学模型。
2.3 吸附等温线
为了评估独居石对氟离子的吸附能力,初步了解其脱氟机理,在上述最佳条件下,获得了独居石脱氟的吸附等温线。图5所示为在pH=1.0,吸附温度70 ℃,吸附时间65 min,氟离子浓度在60~140 mg/L的模拟溶液中独居石脱氟的吸附等温线。
表2 独居石脱氟的动力学模型参数表
Table 2 Kinetic parameters for adsorption of fluoride on monazite

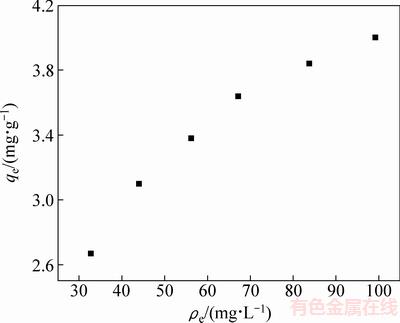
图5 独居石脱氟的吸附等温线
Fig. 5 Adsorption isotherm of fluoride on monazite
表3 独居石脱氟的Frendlich和Langmuir吸附等温线参数表
Table 3 Langmuir and Freundlich isotherm parameters of adsorption of fluoride on monazite

描述吸附过程有两种经典的吸附等温线模型,即Frendlich等温线模型和Langmuir等温线模型,Frendlich模型假定吸附过程发生在非均一的表面,其线性表达式如下:
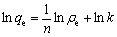 (5)
(5)
式中:ρe为吸附平衡时溶液中氟离子的浓度,mg/L;qe为吸附平衡时氟离子的吸附容量,mg/g;k和n(>1)为Frendlich常数,是经验常数。
Langmuir模型表示吸附过程为单分子层吸附,其线性表达式如下:
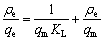 (6)
(6)
式中:qm为理论最大吸附容量,mg/g;KL为Langmuir平衡常数。
表3所列为两个等温线模型拟合后计算得到的参数,图6所示为Frendlich等温线模型和Langmuir等温线模型的拟合图。计算得出的Langmuir模型和Frendlich模型的相关系数分别为0.9992和0.9752,这表明在所研究的溶液体系中,独居石吸附氟离子更符合Langmuir吸附等温线模型,吸附过程为单分子层吸附。通过计算,得出独居石的最大理论吸附容量为5.29mg/g。
2.4 独居石的吸附机理
图7所示为独居石脱氧前后的XPS谱。由图7独居石脱氟前后的光电子能谱图可知,吸附后,在684.74 eV处出现了F1s的元素峰,这表明氟离子已经吸附于独居石的表面。从图中还可以看出,独居石吸附氟离子前后,元素铈的光子能量峰发生了变化:吸附前Ce3d的峰在885.79 eV;吸附后,变成了885.7 eV,Ce3d的峰减少了0.09 eV。产生这种变化的原因是氟离子与金属铈离子发生了静电吸引作用而使相应的峰发生位移,上述结果表明,铈元素的存在使得独居石具有良好的脱氟能力。
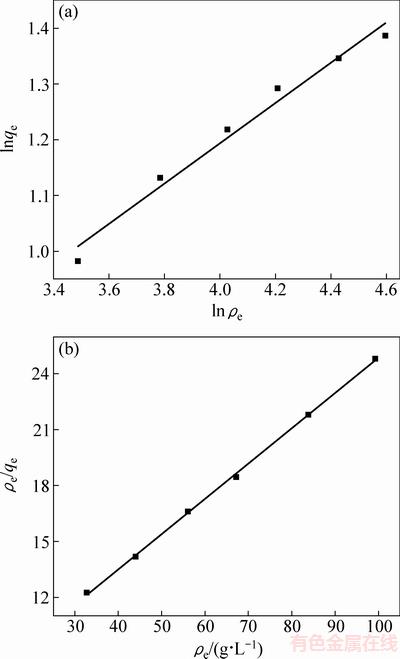
图6 独居石脱氟的Frendlich等温线和Langmuir等温线
Fig. 6 Freundlich isotherm fitting plot (a) and Langmuir isotherm fitting plot (b) of monazite

图7 独居石脱氟前后的XPS谱
Fig. 7 XPS spectra of monazite before and after adsorption
由于独居石中的磷酸盐(以CePO4为主)在酸性溶液中会分解,且随着温度的升高,分解量逐渐增多,因而游离的Ce3+增多,与溶液中的氟离子作用,参与反应(5)生成难溶沉淀CeF3;同时,由反应(6)看出,溶液中HF与H+和F-是处于动态平衡的,且其分解过程是一个吸热过程,温度升高,溶液中的HF离解出更多的F-,使溶液中游离的F-浓度增加,更有利于氟的脱除。因而随温度升高,氟离子浓度是逐渐降低的。
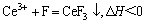 (7)
(7)
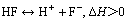 (8)
(8)
但是,另一方面,生成CeF3的反应为放热反应,温度升高会抑制反应的进行,而且温度越高,更多的CeF3和溶液中的一些难溶性的硫酸盐(如CaSO4)析出,覆盖在独居石表面阻碍脱氟反应的进行,所以随温度的升高,氟离子浓度降低的趋势变缓。继续提高温度独居石的吸附效果并没有大的提升,所以,选择70 ℃作为独居石吸附的最佳温度。
另外,独居石在硫酸锌溶液中随酸性增强解离出的铈离子越多,因而pH越低,则有更多的稀土元素Ce解离出来,直接与氟离子结合形成CeF3。因此,随着硫酸锌溶液pH的降低,独居石的脱氟效果越来越好,在pH为1.0时达到最佳,所以为达到较好的脱氟效果,pH应控制在1.0。
3 结论
1) 工艺参数对独居石脱氟效果影响的实验中,可以得出独居石脱氟的最佳条件为:t=65 min,θ=70 ℃,pH=1.0,添加量为25 g/L,在此条件下脱氟率可达46.06%。
2) 独居石脱氟过程符合伪一级动力学模型和Langmuir吸附等温线模型,其等温线模型的线性相关系数为0.9992,体现了独居石对硫酸锌溶液中氟离子为单分子层吸附。同时,得出其最大理论吸附容量为5.29 mg/g。
3) XPS分析证明,氟离子可以被独居石吸附是由于其表面的铈与氟离子相互作用的结果。
REFERENCES
[1] ZHANG Q B, HUA Y X. Kinetic investigation of zinc electrode position from sulfate electrolytes in the presence of impurities and ionic liquid additive [BMIM]HSO4[J]. Materials Chemistry & Physics, 2012, 134(1): 333-339.
[2] MURESAN L M, MAURIN G, ONICIU L, GAGA D. Influence of metallic impurities on zinc electrowinning from sulphate electrolyte[J]. Hydrometallurgy, 1996, 43(1/3): 345-354.
[3] 梅光贵, 王德润, 周敬元. 湿法炼锌学(精)[M]. 长沙: 中南大学出版社, 2004.
MEI Guang-gui, WANG De-run, ZHOU Jin-yuan, WANG Hui. Zinc hydrometallurgy[M]. Changsha: Central South University Press, 2001.
[4] 苏 莎, 陈海清. 湿法炼锌中氟氯去除方法的研究[J]. 湖南有色金属, 2013, 29(1): 40-43.
SU Sha, CHEN Hai-qing. Research on removal of fluoride and chloride in zinc hydrometallurgy[J]. Hunan Nonferrous Metals, 2013, 29(1): 40-43.
[5] 胡一航, 王海北, 王玉芳. 锌冶炼中氟氯的脱除方法[J]. 矿冶, 2016, 25(1): 36-40.
HU Yi-hang, WANG Hai-bei, WANG Yu-fang. Removal of fluorine and chlorine in zinc extraction process [J]. Ming & Metallurgy, 2016, 25(1): 36-40
[6] 谭 青, 李启厚, 刘志宏, 李玉虎, 刘智勇, 刘付朋. 湿法炼锌过程中氟氯脱除技术研究现状[J]. 湿法冶金, 2015(4): 264-269.
TAN Qing, LI Qi-hou, LIU Zhi-hong, LI Yu-hu, LIU Zhi-yong, LIU Fu-peng. Current situation on removal of fluorine and chlorine in zinc hydrometallurgy [J]. Hydrometallurgy of China, 2015(4): 264-269.
[7] XUE T, COOPER W C, PASCUAL R,SAMOTO S. Effect of fluoride ions on the corrosion of aluminium in sulphuric acid and zinc electrolyte[J]. Journal of Applied Electrochemistry, 1991, 21(3): 238-246.
[8] Wu X, LIU Z, LIU X. The effects of additives on the electrowinning of zinc from sulphate solutions with high fluoride concentration[J]. Hydrometallurgy, 2014, 141(2): 31-35.
[9] 谢维新. 湿法炼锌中电解锌溶液脱氟的研究[J]. 广西民族大学学报(自然科学版), 1996(2): 28-32.
XIE Wei-xin. Study on the removal of F- from electrolytic zinc solution used for zinc-making in wet method[J]. Journal of Guangxi University for Nationalities(Natural Science Edition), 1996(2): 26-30.
[10] 万 挺, 梅 晶. 硫酸锌溶液中混凝沉淀降氟实验研究[J]. 广州化工, 2013, 41(24): 84-85.
WAN Ting, MEI Jing. An experimental study of coagulative precipitation process for fluoride removal from zinc sulfate solution[J]. Guangzhou Chemical Industry, 2013, 41(24): 84-85.
[11] 魏 昶, 李存兄, 邓志敢, 李兴彬. 含氟、氯硫酸锌溶液中锌与氟氯分离的方法: 中国, 201010584552.3[P]. 2011-04-20.
WEI Chang, LI Cun-xiong, DENG Zhi-gan, LI Xing-bin. Method to separate zinc with F, Cl from zinc sulfate solution. China, 201010584552.3[P]. 2011-04-20.
[12] 王志斌, 韦国龙, 余 坦, 李世平, 倪常凯. 一种萃取脱脱氟、氯的湿法炼锌方法: 中国, 201210159097.1[P]. 2012-05-21.
WANG Zhi-bin, WEI Guo-long, YU Tan, LI Shi-pin, NI Chang-kai. A method of zinc hydrometallurgy to removal fluoride, chloride by extraction. China, 201210159097.1[P]. 2012-05-21.
[13] 周再兴, 颜文斌, 高 峰, 石美莲, 石爱华. D406螯合树脂对硫酸锌溶液中氟的吸附和解吸性能及动力学研究[J]. 离子交换与吸附, 2010, 26(2): 153-161.
ZHOU Zai-xing, YAN Wen-bin, GAO Feng, SHI Ai-hua. Study on characteristics and kinetics for adsorption and desorption of fluoride from zinc sulfate solution by D406 chelating resin[J]. Ion Exchange and Adsorption, 2010, 26(2): 153-161.
[14] 宋志红. 湿法炼锌工业化离子交换法脱氟氯技术. 中国, 200910042770.1[P]. 2009-07-29.
SONG Zhi-hong. Zinc hydrometallurgy industrialize ions exchange method to removal F, Cl. China, 200910042770.1[P]. 2009-07-29.
[15] MALIYEKKAL S M, SHUKLA S, PHILIP L, NAMBI I M. Enhanced fluoride removal from drinking water by magnesia-amended activated alumina granules[J]. Chemical Engineering Journal, 2008, 140(1/3): 183-192.
[16] RAICHUR A M, BASU M J. Adsorption of fluoride onto mixed rare earth oxides[J]. Separation & Purification Technology, 2001, 24(1/2): 121-127.
[17] TURNER B D, BINNING P, STIPP S L. Fluoride removal by calcite: Evidence for fluorite precipitation and surface adsorption[J]. Environmental Science & Technology, 2005, 39(24): 9561-9568.
[18] GOGOI S, DUTTA R K. Fluoride removal by hydrothermally modified limestone powder using phosphoric acid[J]. Journal of Environmental Chemical Engineering, 2016, 4(1): 1040-1049.
[19] ZHANGS Y, LU Y, LIN X Y, SU X S, ZHANG Y L.Removal of fluoride from groundwater by adsorption onto La(III)-Al(III) loaded scoria adsorbent[J]. Applied Surface Science, 2014, 303(5): 1-5.
[20] 刘争伟, 于枭影, 钟晓聪, 杨 凡, 蒋良兴, 刘芳洋, 赖延请. 含Al、Ca复合脱氟剂在含氟硫酸锌溶液中的脱氟性能[J]. 中国有色金属学报, 2016, 26(5): 1151-1157.
LIU Zheng-wei, YU Xiao-ying, ZhONG Xiao-cong, YANG Fan, JIANG Liang-xing, LIU Fang-yang, LAI Yan-qing. Performance of Ca, Al-containning composite defluoridation agent in zinc sulfate solution[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(5): 1151-1157.
[21] FANG Z, XUE N, LI L B, HONG T, KONG H L, WU J N. Performance and mechanism for de-fluorination by modified aluminum hydroxide in zinc sulfate solution[J]. Journal of Nanoscience and Nanotechnology, 2016, 16(12): 12470-12475.
Adsorption properties of fluorine onto monazite from industrial zinc sulfate solution
LAI Yan-qing, YANG Kai, GUO Wei-chang, YANG Chao, TIAN Zhong-liang, ZHANG Kai, LI Jie
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: Monazite was employed to remove fluorine ions from industrial zinc sulfate solution. The different process parameters, such as adsorption time, adsorption temperature, dosage of adsorbent and pH, were studied. Kinetic and adsorption isotherm characteristics was analyzed and the mechanism of defluorination on monazite was explored preliminarily. The results indicate that fluorine ions are removed by interacting with equilibrium concentration on the surface of monazite. The kinetics data suggests that the process of adsorption can be described by the pseudo-first order kinetic model. The isotherm data are well fitted to the Langmuir isotherm models and the theoretical maximum adsorption capacity is 5.29 mg/g. It is the most efficient when the conditions are as follows: t=65 min, θ=70 ℃, ρ=25 g/L, pH=1. And under this condition, the efficiency can reach up to 46.06%.
Key words: Monazite; industrial zinc sulfate solution; defluorination
Foundation item: Project(51334002) supported by the National Natural Science Foundation of China
Received date: 2017-07-03; Accepted date: 2017-12-06
Corresponding author: TIAN Zhong-liang; Tel: +86-731-88830474; E-mail: tianzhongliang@csu.edu.cn
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(51334002)
收稿日期:2017-07-03;修订日期:2017-12-06
通信作者:田忠良,教授,博士;电话:0731-88830474;E-mail:tianzhongliang@csu.edu.cn