磨矿环境和晶格杂质对锂辉石浮选的影响
来源期刊:中国有色金属学报(英文版)2019年第7期
论文作者:朱广丽 王毓华 王旭明 Jan D. MILLER 卢东方 郑霞裕 赵悦豪 郑海涛
文章页码:1527 - 1537
关键词:磨矿;晶格取代;金属杂质;DFT 计算;锂辉石;解理;浮选
Key words:grinding; lattice substitution; metal ion impurities; density functional theory (DFT) calculation; spodumene; cleavage; flotation
摘 要:研究油酸钠体系中磨矿对锂辉石浮选行为的影响。不同磨矿环境导致锂辉石表面暴露的金属活性位点的含量有差异,从而显著影响锂辉石的可浮性。酸处理后金属活性位点脱离锂辉石表面,使得锂辉石的可浮性下降,进一步表明金属活性位点对锂辉石浮选的重要作用。金属离子杂质可能来源于磨矿环境或晶格杂质。密度泛函理论(DFT)计算表明,杂质铁、钙质点主要是以晶格取代的形式存在于锂辉石表面,镁难以发生晶格取代。与钙和镁相比,铁更易于与油酸钠发生相互作用。扫描电镜结果表明,锂辉石的粒度和解理特性不同,导致其暴露晶面的差异。在不同磨矿环境下,粒度、解理特性和金属离子杂质对油酸钠捕收剂浮选锂辉石起着重要作用。
Abstract: The effect of grinding on the spodumene flotation was investigated. The flotation response of spodumene ground by different mills was different, due to the variation of metal ions on spodumene surfaces caused by grinding environments and/or impurities. The samples were subjected to acid pickling treatment to remove most of the metal ions from the surfaces, and then all samples showed the same poor flotation response, which confirmed the significance of surface metal ions. Metal ion impurities may come from both grinding environments and lattice substitutions in spodumene. Density functional theory (DFT) calculation revealed that Fe and Ca could exist as lattice substitutions on the spodumene surface while Mg substitution is unlikely to occur. Furthermore, Fe is considered to be active site for the absorption of sodium oleate on the spodumene surface. Morphology analysis showed differences in particle size and shape for samples ground by different mills, resulting in different amounts of exposed surfaces. The particle size, cleavage characteristics caused by grinding environments, and metal ion impurities originated from grinding and isomorphous substitutions, play significant roles in the chemisorption of collector on the spodumene surface.
Trans. Nonferrous Met. Soc. China 29(2019) 1527-1537
Guang-li ZHU1,2, Yu-hua WANG1, Xu-ming WANG2, Jan D. MILLER2,Dong-fang LU1, Xia-yu ZHENG1, Yue-hao ZHAO1, Hai-tao ZHENG1
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. College of Mines and Earth Sciences, University of Utah, Salt Lake City 84112, USA
Received 7 July 2018; accepted 25 January 2019
Abstract: The effect of grinding on the spodumene flotation was investigated. The flotation response of spodumene ground by different mills was different, due to the variation of metal ions on spodumene surfaces caused by grinding environments and/or impurities. The samples were subjected to acid pickling treatment to remove most of the metal ions from the surfaces, and then all samples showed the same poor flotation response, which confirmed the significance of surface metal ions. Metal ion impurities may come from both grinding environments and lattice substitutions in spodumene. Density functional theory (DFT) calculation revealed that Fe and Ca could exist as lattice substitutions on the spodumene surface while Mg substitution is unlikely to occur. Furthermore, Fe is considered to be active site for the absorption of sodium oleate on the spodumene surface. Morphology analysis showed differences in particle size and shape for samples ground by different mills, resulting in different amounts of exposed surfaces. The particle size, cleavage characteristics caused by grinding environments, and metal ion impurities originated from grinding and isomorphous substitutions, play significant roles in the chemisorption of collector on the spodumene surface.
Key words: grinding; lattice substitution; metal ion impurities; density functional theory (DFT) calculation; spodumene; cleavage; flotation
1 Introduction
Lithium and several of its compounds, as critical and strategic materials, have been widely used in a variety of applications such as the battery and fuel cell industry, due to their excellent attributes [1,2]. Global demand for lithium is expected to increase, due to growing application for electric vehicles, portable electronics, and grid storage systems [3,4]. Therefore, the recovery of lithium from available economic resources including brines and ore deposits which contain varied silicate minerals, such as spodumene, has drawn wide attention. In particular, spodumene (LiAl[SiO3]2), the richest lithium-bearing minerals in nature, is one of the most widely exploited minerals from lithium-rich pegmatite ores.
Froth flotation is a widely applied industrial method for the beneficiation of spodumene using traditional fatty acid collectors, such as oleate and soaps, or mixed reagents [5-7]. The surface chemistry of aluminosilicate minerals is found to account for the selective separation in the industrial flotation of spodumene ores using oleate as collector. Oleate is chemically adsorbed on spodumene surface, involving the association of the carboxylate group of oleate and Al sites on the surface. Therefore, the nature of atomic sites on spodumene surfaces, which bring about anisotropic surface hydrophobicity, is responsible for adsorption states of oleate collector on the spodumene surfaces [6,8-11]. Researchers proposed that the (110) surface of spodumene is more in favor of the chemisorption of oleate collector than the (001) surface, since there are two and one Al—O broken bonds per Al site on the (110) and (001) surfaces, respectively. Thus, MOON [8] recommended that the impact comminution mechanism that preferentially produces the (110) cleavage surface of spodumene should be employed. That is, various crushing and grinding methods should be performed to produce different cleavage and fracture characteristics of minerals so the wettability and hydrophobicity of spodumene may be modified.
In practice, however, it should be noted that spodumene is difficult to float in the presence of NaOL alone, while adsorption of NaOL is enhanced greatly when multivalent metallic cations such as Fe3+, Ca2+, and Mg2+ are used as activators. Studies involving effects of multivalent metallic cations on flotation were carried out by many researchers [12-15]. YU et al [13] studied the mechanism of spodumene activation by Ca2+ using quantum chemical calculations, and proposed that the precipitation of Ca(OH)+ and Ca(OH)2 on spodumene surface contributed to the activation of spodumene flotation. In alkaline solutions, Ca2+ and Mg2+ ions readily form hydroxy complexes and precipitates, which were adsorbed on the spodumene surface and formed oleate complexes. Therefore, the collector adsorption is enhanced [16]. Also, spodumene surface was found to transform from polar to non-polar in the presence and absence of Fe3+ activator using NaOL as a collector [17]. MOON [8] suggested to use steel grinding as opposed to autogenous grinding as Fe is rich in steel mills.
Apart from operating water, iron mills, and addition of cations during the flotation process, multivalent metallic cations may inherently exist in spodumene as lattice impurities. Spodumene is a typical monoclinic pyroxene mineral with single-chain structure. These chains of silicate tetrahedra are laterally bound together through bonding with Al (M1 site) and Li (M2 site) in octahedral coordination. It is common to find that in the crystal structure of spodumene, some Li+ ions are substituted by Na+ and K+ ions, and some Al3+ ions are replaced by other impurities including Fe3+, Cr3+, Mn2+ ions etc [18-21]. Hence, natural spodumene crystals vary in color from colorless, pink (kunzite) to green (hiddenite), depending on impurity types and concentrations. Lattice impurities have important effects on electronic structures and flotability of minerals. For example, flotability of spodumene with different colors is different due to Fe impurity concentrations [22]. In sulphide minerals, for instance, sphalerite with 14 kinds of lattice impurities studied by the DFT calculation showed that impurities had various effects on the oxidization of sphalerite and the reactivity of xanthate with sphalerite [23]. Therefore, the study on effects of impurities in the mineral lattice is necessary to obtain a better understanding of mineral performance in flotation.
In the present study, the effect of grinding using different mills on spodumene flotation was investigated, and the ground samples were characterized by micro-flotation, particle size distribution, XPS, and SEM measurements. The perfect cleavage (110) surface with or without substitutions was studied using DFT calculation to see if the surface metal ion impurities were possible from lattice substitutions. These results are significant in understanding the effects of grinding environment and metallic element impurities on spodumene flotation using sodium oleate as a collector.
2 Experimental
2.1 Sample preparation and reagents
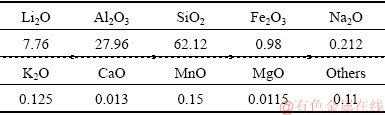
The pure samples of spodumene were hand-picked from Koktokay Rare Metallic Mine, Xinjiang, China, and crushed to be less than 3 mm. A high intensity dry magnetic separator was used to eliminate metal impurities. The chemical composition of samples is shown in Table 1. The collector sodium oleate (>97.0 wt.%, TCI) was used in the micro-flotation experiments. pH modifiers for the flotation system were HCl and NaOH solution. Deionized (DI) water from a Millennium water purification system with a minimum resistivity of 18.2 MΩ·cm was used in all experiments.
Table 1 Chemical composition of spodumene samples (wt.%)
2.2 Grinding experiments
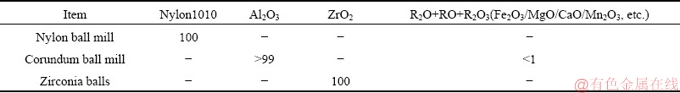
The grinding experiments were carried out in a 105 mm × 140 mm nylon ball mill (small nylon mill), a 105 mm × 140 mm corundum ball mill (small corundum mill), and a 165 mm × 203 mm corundum ball mill (large corundum mill). The rotation speed of the mills was fixed at 30 r/min. The grinding media were zirconia balls with various diameters (10, 15, 20, and 25 mm). The chemical compositions of ball mills and zirconia balls are presented in Table 2. 80 g spodumene samples were mixed with 40 mL DI water, and ground for 5 min for each run. Then, the ground products were sieved to get a size range of less than 105 μm. Finally, the screen residue was returned for further grinding. This process was repeated until the samples with a size less than 105 μm were sufficient for the following experiments. Afterwards, ground samples were further sieved to 23-105 μm. A fraction of the 23-105 μm samples was wet screened to obtain the products in 75-105 μm, 45-75 μm, and 23-45 μm size ranges. The samples in different size ranges were rinsed using DI water, dried, and weighed to determine the particle size distribution.
Table 2 Chemical composition of mills and balls (wt.%)
2.3 Acid pickling method
The acid pickling treatment for the ground samples was carried out to “clean” the surface of the samples. Spodumene samples were placed in a beaker filled with 3% HCl solution. The system was stirred using a magnetic stirrer for 40 min, and then stood for 1 min, after which the upper liquid was decanted. Subsequently, DI water was added to the system, and the system was stirred for 40 min again and then stood for 1 min again, and the supernatant was decanted. This procedure was repeated until the pulp pH was neutral. After the supernatant was decanted, the sediments were filtered and dried for micro-flotation experiments and XPS measurements.
2.4 Micro-flotation experiments
Micro-flotation experiments were conducted on a laboratory self aspirating flotation machine at 1700 r/min. For each test, 2 g samples with or without acid pickling were placed separately in a 20 mL plexiglass cell, and then the cell was filled with DI water. The pH of the system was adjusted by HCl or NaOH solutions, and then the system was conditioned for 2 min, followed by the addition of the 6×10-4 mol/L sodium oleate. After the system was conditioned for 2 min, the froth products were collected for 3 min. All the flotation experiments were performed at room temperature of (25±1) °C.
2.5 XPS measurement
The XPS analysis was performed using a Thermo Scientific ESCALAB 250Xi with monochromatic Al Kα radiation, a wide spectrum collected from 1350 to 0 eV with a passing energy of 100 eV and a step size of 1 eV. The spectra were recorded at a 1 eV resolution. For high-resolution analysis, the passing energy was 30 eV with a 50 meV step, performed for carbon (C 1s), oxygen (O 1s), silicon (Si 2p), aluminum (Al 2p), lithium (Li 1s), iron (Fe 2p), calcium (Ca 2p), and magnesium (Mg 1s).
2.6 Scanning electron microscope (SEM) analysis
The spodumene samples were subjected to SEM (JSM-6490LV) analysis for the characterization of morphology. Samples were coated with Pt to make the samples conductive.
2.7 DFT calculation
Crystal structure of spodumene was built using lattice parameters from the American Mineralogist Crystal Structure Database. Geometry optimizations of the crystal structure were calculated using the Cambridge Serial Total Energy Package (CASTEP) that employs the density functional theory plane-wave pseudopotential method to perform first-principles quantum mechanics calculations, which enables the exploration of the properties of crystals and surfaces in materials [24]. The exchange-correlation functional local density approximation (LDA) was employed and the Ceperley- Alder and Perdew-Zunger (CA-PZ) was used as exchange-correlation potentials for the LDA [25-27]. The BFGS method using line search was used for the geometry optimization. The Pulay Density Mixing method was applied as the self-consistent electronic minimizer with a convergence tolerance of 1.5×10-6 eV/atom. The parameters of the optimized crystal structure are a=9.486922 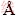 , b=8.237576
, b=8.237576 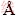 , c=5.181515
, c=5.181515  , β=110.7417°, which are consistent with those reported experimentally [28].
, β=110.7417°, which are consistent with those reported experimentally [28].
The (110) surface was created from the bulk spodumene crystal and optimized. After the geometry optimization of the crystal surface, a 2×2×1 perfect spodumene (110) surface with 20  vacuum slab was modeled. The perfect spodumene (110) surface is chosen and optimized on the basis that the one which has the lowest surface energy is taken as the cleavage (110) surface. The surface energy (Esurf) was computed based on the Eq. (1):
vacuum slab was modeled. The perfect spodumene (110) surface is chosen and optimized on the basis that the one which has the lowest surface energy is taken as the cleavage (110) surface. The surface energy (Esurf) was computed based on the Eq. (1):
 (1)
(1)
Where Eslab and Ebulk are the energies of the surface slab and the bulk crystal, respectively; Nslab and Nbulk are the atom number of the surface slab and the bulk crystal, respectively; A is the surface area.
The (110) surfaces with lattice substitutions were built by substituting one impurity atom (Fe, Ca, and Mg) for one Al atom on the surface, or by substituting one impurity atom (Ca and Mg) for one Li atom and simultaneously one Al atom substituting one Si on the surface, or deleting Al or Li atoms. The atoms of the top four layers were relaxed, and the other layers were fixed. The model of the spodumene (110) surface is depicted in Fig. 1. The energy of an impurity atom X (Fe, Ca, and Mg) for one Y atom of spodumene is defined as follows [29]:
△E=Esubstitution+EY-E-EX (2)
where Esubstitution and E represent the total energies of the (110) surface with and without X substitution, respectively; EY, EX are the total energy of Y atoms and X atoms, respectively.
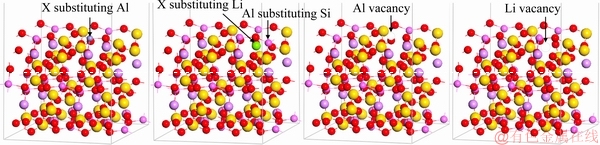
Fig. 1 Models of spodumene (110) surface with different substitutions and vacancies
The vacancy formation energy of spodumene (110) surface is
△E=Evacancy+EX-E (3)
where Evacancy and E are the total energies of the (110) surface with and without vacancy, respectively; EX is energies of Al or Li atoms.
3 Results and discussion
3.1 Particle size distribution
The particle size distribution of the samples ground by different mills is illustrated in Fig. 2. The proportion of samples ground by the large corundum mill in the size range of 75-105 μm is 20.83%, which is the lowest compared to that of the other two samples ground by the small corundum (37.13%) and nylon mills (40.71%) in the same size range. The proportions of the samples ground by the large corundum mill, the small corundum mill, and the small nylon mill in the size range of 45-75 μm are 28.52%, 32.55%, and 30.36%, respectively. The sample ground by the large corundum mill has the highest distribution (50.65%) in the size range of 23-45 μm, and in terms of the samples ground by the small mills, the proportions in this size range are 30.32% and 28.93%, respectively. It is obvious that on the whole, the particle size of samples ground by the large corundum mill is finer than that of samples ground by the small corundum and nylon mills.
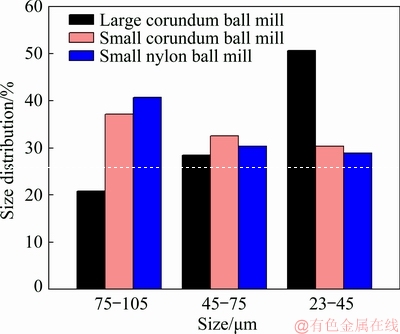
Fig. 2 Particle size distribution of spodumene samples ground by different mills
3.2 Micro-flotation
Micro-flotation experiments were done to compare the flotation response of spodumene ground by various mills as well as with different size ranges. Figure 3 shows the flotation recovery of spodumene samples ground by the large corundum ball mill, the small corundum ball mill, and the small nylon ball mill with different size ranges. Figure 4 displays the flotation results for spodumene in the size range of 23-105 μm with or without acid pickling treatment. In general, the change of flotation recovery follows the same trends as pH changes for all of the spodumene samples. The recovery increases as the pH values increase, and reaches a peak around pH 8-9, before declining as the pulp pH increases further. As can be seen from Fig. 3, the flotation response of spodumene follows the order, 75-105 μm > 45-75 μm > 23-45 μm for all the samples ground by the different mills. The flotation recovery of spodumene ground by the large corundum ball mill in the size range of 75-105 μm is slightly lower than that of samples ground by the small nylon mill and small corundum mill. In terms of the whole size range of 23-105 μm, the recovery of spodumene ground by the small corundum mill and small nylon mill is similar, both of which are higher than that of spodumene ground by the large corundum mill. However, the flotation recovery of all samples decreases to be almost the same within the pH ranges after the acid pickling treatment, demonstrating that all the ground samples with acid pickling treatment have poor flotation response (Fig. 4).
The micro-flotation results show that the flotability of the samples declines along with the decrease of the particle size, similar results have been reported previously [30]. Spodumene is a monoclinic crystal that is primarily composed of the cleavage (110) side surface and the (001) basal surface. The (110) surface has been confirmed to be more favored than the basal (001) surface for the chemisorption of oleate because of its higher unsaturated Al sites. With the decrease of the particle size, the relative content of (110) surfaces decreases, resulting in the decline of the flotability. In addition, the flotation recovery of the sample ground by the large corundum mill is the lowest among the three samples in the whole size range of 23-105 μm. As indicated by particle size distribution results, sample ground by the large corundum mill has the lowest content of the coarser 75-105 μm size fraction which has relative better flotability compared with the other two size ranges. Thus, the lower proportion of the coarser 75-105 μm size fraction of samples of the whole size fraction ground by the large corundum mill may be one of the reasons for its poor flotation response compared with the other two samples.
However, it should be noted that even for the same narrow particle size fraction, the flotation recovery of the samples ground by the large corundum mill is slightly lower than that of samples ground by the small corundum and nylon mills, as shown in Fig. 3(d). All samples possess similar poor flotation response within the studied pH ranges after acid pickling, as shown in Fig. 4. To understand the reason for this, the surface properties of samples were analyzed in the following sections.
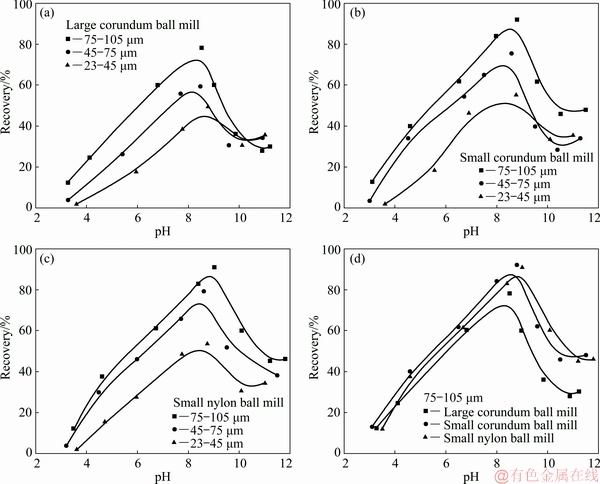
Fig. 3 Flotation of spodumene ground by different ball mills with different size ranges as function of pH, using 6×10-4 mol/L sodium oleate as collector
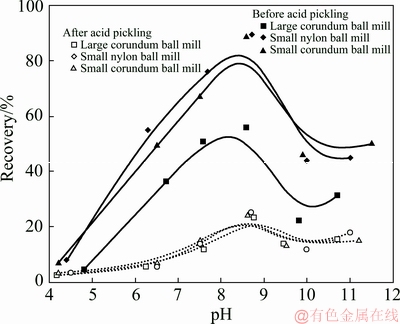
Fig. 4 Flotation of spodumene ground by different mills in size range of 23-105 μm before and after acid pickling as function of pH, using 6×10-4 mol/L sodium oleate as collector
3.3 XPS and SEM analysis
It is widely accepted that sodium oleate mainly interacts with Al sites on the spodumene surfaces [6,9-11,31-34]. To characterize the chemical composition of the surfaces of spodumene ground by different mills, the X-ray photoelectron spectroscopy (XPS) measurement of samples was carried out, and the results are shown in Fig. 5. Except for the elements pertaining to spodumene and the so-called advantageous or rubbish carbon, the presence of Fe, Ca, and Mg elements, however, was identified. The relative mole concentration of elements on the different spodumene surfaces was calculated according to the spectra as presented in Tables 3 and 4.
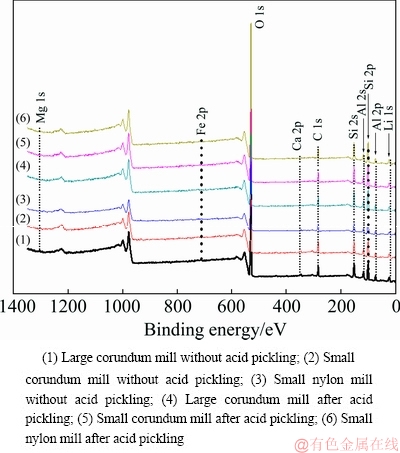
Fig. 5 XPS spectra of spodumene samples ground by different mills
Without the acid pickling, both surfaces of spodumene samples ground by the large and small corundum mills contain Fe, Ca, and Mg impurities, whereas samples ground by the small nylon mill do not contain Mg impurities. The surface of spodumene ground by the large corundum mill has less total metal ions of Al, Ca, Mg, and Fe, which might be an essential factor contributing to its poor flotation response. Besides, spodumene ground by the nylon mill, which has less metal ion impurities of Ca, Mg, and Fe, floats better than that by the large corundum mill with more metal ion impurities, and this might be due to its higher relative concentration of Al and Ca. The difference in chemical composition of the surface might be mainly due to particle shape and anisotropic surface property apart from foreign impurities introduced by the barrel and balls of mills. By comparing the three SEM images in Fig. 6, it can be found that in general, the particle size of the samples ground by the large corundum mill is smaller than that of spodumene ground by the small corundum and nylon mills, which is consistent with the particle size distribution results. Furthermore, the particle shapes of spodumene ground by the large corundum mill are long, flat and prismatic, some of which are bladed. However, particle shapes of spodumene ground by the small corundum and nylon mills are similar: flaky, wider than that of samples ground by large corundum mill. The main exposed perfect cleavage (110) surface, along with the basal surface (001) and the fracture surface (010) have different atomic site distributions, and therefore these surfaces have different properties. We have studied that the (110) and (010) surfaces are more in favor of the adsorption of sodium oleate than (010) and (001) surfaces because of their higher density of Al sites and Al—O broken bonds [11]. The difference in the shape of samples could lead to difference in the relative content of exposed crystal surfaces, which results in the small difference in flotation recovery of samples in the size fraction of 75-105 μm.
Table 3 Chemical composition determined by XPS measurements for spodumene ground by different mills without acid pickling
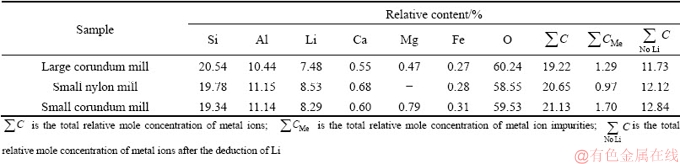
Table 4 Chemical composition determined by XPS measurements for spodumene ground by different mills after acid pickling
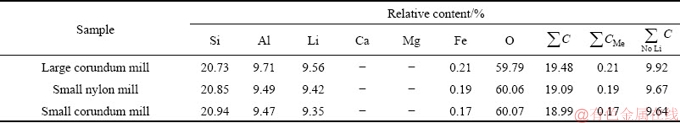
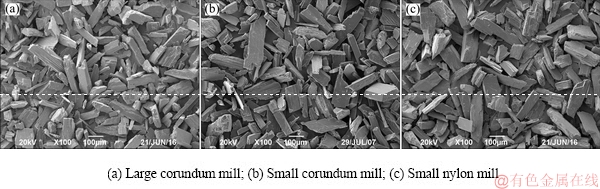
Fig. 6 SEM images of spodumene ground by different mills
After the acid pickling, for all samples, the relative concentrations of Al and metal ion impurity elements all decreased, while the relative concentration of Li increased. Meanwhile, flotation response of all the samples became poor after acid pickling, which may be due to that Li sites are not capable of interacting with the carboxylate group of oleate, since the main interacting sites on the spodumene surfaces with sodium oleate are multivalent metal ion sites. Comparing the samples ground by different mills, although the samples ground by the large and small corundum mills have more metal ion impurities than that by the nylon mill, samples ground by the small corundum and nylon mills floated better than that by the large corundum mill, because of higher total metal ion content on the surface resulting from shape factors. After the acid pickling, the reduced metal ion impurities on the sample surfaces gave rise to poor flotation response for all samples. Therefore, it can be concluded that the root cause of the difference in flotation response is the metal ion content on sample surfaces, which depends on both metal ion impurities, the particle size distribution, and the cleavage characteristics of spodumene under different grinding conditions.
Besides, it can be inferred that metal ion impurities come from spodumene, which exist as lattice substitution, or grinding environments. Mg element was not found on the surfaces of samples ground by the nylon mill which contains no metal ions. All the metal ion impurities were found on the surfaces of samples ground by the corundum mills. These results suggest that Fe and Ca impurities may be partially from lattice impurities, while the majority of Mg may come from mills. Quantum chemistry calculation needs to be further done to confirm this inference.
3.4 Computational analysis
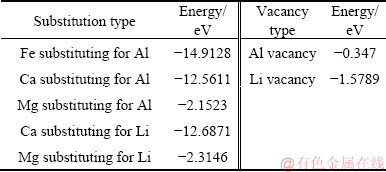
Though many studies reported that metal ions such as Fe, Mn, and Cr are common lattice impurities substituting Al sites in spodumene [18-21], few studies are reported on lattice substitution of spodumene by DFT calculations. In order to evaluate whether the metal ion impurities could be lattice impurities in spodumene crystal, as well as the influence of lattice substitution on electronic properties and flotation response of spodumene, the (110) surfaces of spodumene bearing Fe, Ca, Mg substitutions, and Li, Al vacancies, are modeled using computer simulation techniques. The energies of the spodumene (110) surface with substitutions and vacancies are shown in Table 5. The more negative values of energies are, the lattice substitutions or vacancies occur more readily. The substitution energies of Fe, Ca and Mg were negative, suggesting that these atoms exist on the (110) spodumene surface. Nevertheless, the substitution energies for Mg impurity were higher, suggesting that the incorporation of Mg may need higher temperature and/or higher pressure during crystallization. Formation energies of Li and Al vacancy on the spodumene (110) surface were negative values, manifesting that Li and Al are readily dissolved in solutions. In addition, the substitution of Fe for Al atom was the most energetically favorable, corresponding well with the fact that Fe-bearing spodumene is very common in nature.
Table 5 Energies of spodumene (110) surface with different substitutions and vacancies
The partial density of states (PDOS) of the spodumene (110) surface with substitutions and vacancies calculated by the CASTEP module are presented in Fig. 7. The Fermi energy was set as 0 eV (Ef=0). As seen in Figs. 7(a-c), the surface state can be observed at the Fermi level for the Fe substitution surface, which is composed of Fe 4s and Fe 3p orbitals, and Ca has little contribution, with Ca 3d orbital, while Mg has no contribution to the DOS around the Fermi level, indicating that the activity of Fe is stronger than Ca and Mg, and Fe is apt to act as active sites for the adsorption of anionic collectors (sodium oleate) on the spodumene surface. With respect to Ca and Mg substituting for Li, as shown in Figs. 7(d) and (e), both Ca and Mg have no contribution to the DOS around the Fermi level, indicative of weak activity. With respect to (110) surface with vacancies, Al and Li contribute little to the DOS around the Fermi level, showing that reactivity of Al and Li on the (110) surface is poor, which is consistent with the poor flotation response after acid pickling.
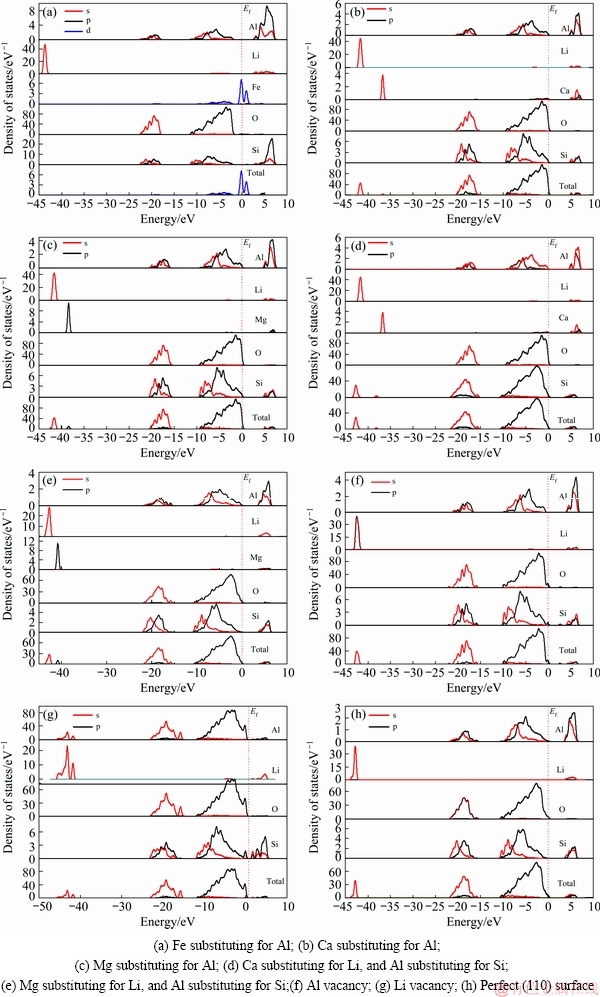
Fig. 7 PDOS of Li, Al, Si, O, and impurity atoms on spodumene (110) surface
As shown in Table 6, the bond populations of Fe—O2, Ca—O2, and Mg—O2 are 0.39, 0.12, and -1.54, respectively, indicating that the covalent proportion in the bond of Fe—O2 is very strong but weak for Ca—O2, manifesting a stronger bond of Fe—O2 formed. However, the bond population of Mg—O2 is negative, indicating that Mg and O2 atoms are in an anti-bonded state [35-37]. Also, in the case of Ca and Mg substituting for Li, bond populations of Ca—O are positive while bond populations of Mg—O are negative, suggesting that Ca and O could be bonded while Mg and O atoms are in anti-bonded states. In other words, Mg impurity is less likely to substitute the Al or Li sites in spodumene crystal, while Fe and Ca are possible lattice impurities, combining the analysis of PDOS results.
Table 6 Mulliken atomic populations of metal ion impurities and Mulliken bond population analysis
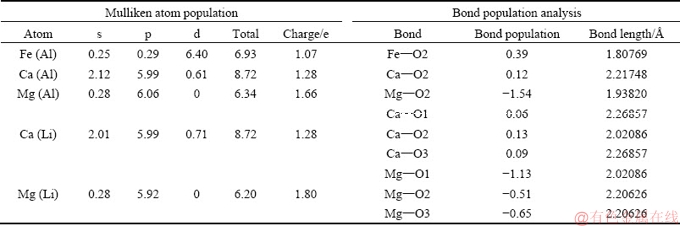
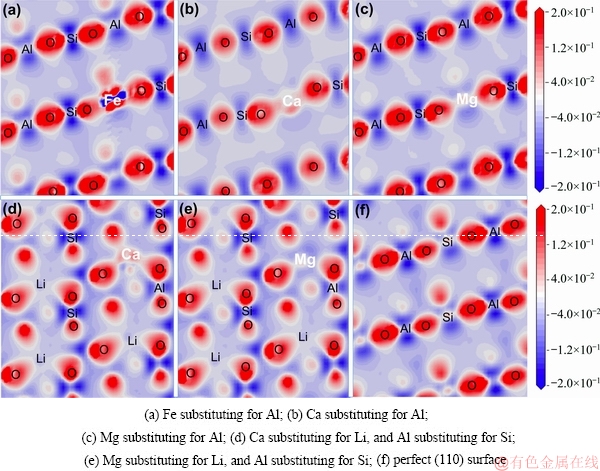
Fig. 8 Electron density difference of spodumene (110) surface with substitutions
The electron density differences between the O atom and Fe, Ca, and Mg confirmed the inference. As shown in Fig. 8, there is large electronic density between Fe and O atoms, and the overlap of the electron cloud between Fe—O2 is greater than Ca—O, revealing that the interaction between Fe and O is relatively strong. However, the electronic density difference between Mg and O is almost negative, indicating that the interaction of Mg with O is very weak.
Based on the calculation, it can be concluded that it is highly likely that Fe is an impurity of spodumene crystal, and Ca might be an impurity of spodumene crystal as well, whereas Mg is the least likely to be the lattice impurity. This conclusion is consistent with the XPS results that there is no Mg on the surface of samples ground by nylon mill which contains no metallic elements. In addition, the activity of Fe is stronger than Ca and Mg, and thus Fe is more apt to act as active sites for the absorption of sodium oleate on the spodumene surface. The reactivity of Al and Li on the (110) surface is poorer, compared to that of Fe. The (110) surface with Al or Li vacancy is readily to be formed according to the calculation. Thus, the metallic elements may partially dissolve into the solution after acid pickling, resulting in poor flotation response of spodumene samples.
4 Conclusions
1) The flotation response of spodumene becomes better in the sequence of 75-105 μm > 45-75 μm > 23-45 μm size range for the samples ground by different mills. The flotation recovery of samples ground by the large corundum mill is lower than that of the samples ground by the small corundum and nylon mills for the same particle size range. All the samples possess poor flotation response after acid pickling.
2) The difference in flotation response was majorly due to the variation of metal ions on the spodumene surface as revealed by XPS results. The less total metal ions on the surfaces of spodumene ground by the large corundum mill may be the main reason for its poor flotation response as compared to samples ground by the other two mills. After acid pickling, the relative concentrations of Al and metal ion impurities all decreased, which seems to contribute to the poor flotation response of all the samples.
3) The particle size distribution indicates that samples ground by the large corundum mill have a higher percentage of fine size. SEM images show that spodumene ground by the large corundum has a finer particle size and the particles are in a long, flat and prismatic shape. Therefore, the particle size distribution and particle shape account for the distinct relative contents of exposed surfaces in the samples, and the variation of metal ions on the surfaces.
4) Computational results demonstrate that Fe and Ca could be lattice substitutions in spodumene while Mg substitution is unlikely to occur. In addition, Fe is considered to act as active sites for the absorption of sodium oleate on the spodumene surface. The (110) surface with Al or Li vacancy is readily to be formed according to the calculation. Therefore, the metallic elements on the spodumene surfaces may partially dissolve into solution after acid pickling, resulting in poor flotation response of spodumene samples.
References
[1] KEITH E. Critical metals handbook: Lithium [M]. Oxford, UK, John Wiley & Sons, 2013: 230-260.
[2] CHU S. Critical materials strategy [M]. DIANE Publishing, 2011: 69-95.
[3] GUNTHER M, LARS R, MICHAEL H, MARTIN B. Lithium market research–global supply, future demand and price development [J]. Energy Storage Materials, 2017, 6: 171-179.
[4] BASUDEV S. Recovery and recycling of lithium: A review [J]. Separation and Purification Technology, 2017, 172: 388-403.
[5] XIE Zhen-fu, WANG Yu-hua, YU Fu-shun, TANG Zi-jun, ZHU Guang-li. Reviews of flotation research on pegmatite spodumene ores [J]. Chinese Journal of Rare Metals, 2013, 37(4): 641-649.
[6] XU Long-hua, HU Yue-hua, WU Hou-qin, TIAN Jia, LIU Jing, GAO Zhi-yong, WANG Li. Surface crystal chemistry of spodumene with different size fractions and implications for flotation [J]. Separation and Purification Technology, 2016, 169: 33-42.
[7] LI Si, LU Dong-fang, CHEN Xing-hua, ZHENG Xia-yu, LI Xu-dong, CHU Hao-ran, WANG Yu-hua. Industrial application of a modified pilot-scale Jameson cell for the flotation of spodumene ore in high altitude area [J]. Powder Technology, 2017, 320: 358-361.
[8] MOON K S. Surface and crystal chemistry of spodumene and its flotation behavior [D]. Berkeley: University of California, 1986, 11-73.
[9] BEENA R, SATHISH P, JYOTSNA T, MOON K S, FUERSTENAU D W. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals [J]. Journal of Colloid and Interface Science, 2011, 362(2): 510-516.
[10] MOON K S, FUERSTENAU D W. Surface crystal chemistry in selective flotation of spodumene (LiAl[SiO3]2) from other aluminosilicates [J]. International Journalof Mineral Processing, 2003, 72: 11-24.
[11] ZHU Guang-li, WANG Yu-hua, LIU Xiao-wen, YU Fu-shun, LU Dong-fang. The cleavage and surface properties of wet and dry ground spodumene and their flotation behavior [J]. Applied Surface Science, 2015, 357: 333-339.
[12] WANG Yu-hua, YU Fu-shun. Effects of metallic ions on the flotation of spodumene and beryl [J]. Journal of China University of Mining and Technology, 2007, 17(1): 35-39.
[13] YU Fu-shun, WANG Yu-hua, WANG Jin-ming, XIE Zhen-fu. Investigation on different behavior and mechanism of Ca(II) and Fe(III) adsorption on spodumene surface [J]. Physicochemical Problems of Mineral Processing, 2014, 50(2): 535-550.
[14] LI En-ze, DU Zhi-ping, WANG Bo, CHENG Huai-gang, CHENG Fang-qin. Flotation mechanism of soluble salts with high ionic strength [J]. Progress in Chemistry, 2016, 28(9): 1417-1425.
[15] YU Fu-shun, WANG Yu-hua, WANG Jin-ming, XIE Zhen-fu, ZHANG Lei. First-principle investigation on mechanism of Ca ion activating flotation of spodumene [J]. Rare Metals, 2014, 33(3): 358-362.
[16] LIU Wei-jun, ZHANG Shi-qiu, WANG Wei-qing, ZHANG Jie, YAN Wu, DENG Jie. The effects of Ca (II) and Mg (II) ions on the flotation of spodumene using NaOL [J]. Minerals, 2015, 79: 40-46.
[17] ZHANG Jie, WANG Wei-qing, LIU Jing, HUANG Yang, FENG Qi-ming, ZHAO Hong. Fe(III) as an activator for the flotation of spodumene, albite, and quartz minerals [J]. Minerals Engineering, 2014, 61: 16-22.
[18] FUJII A T, ISOTANI S. Comparative study of five varieties of spodumene through optical absorption [J]. Pesquisa Agropecuária Brasileira, 1983, 16(03): 1-14.
[19] ISOTANI S, KAZUNORI W, AKIYOSHI M, WALDEMAR B. UV optical absorption spectra analysis of spodumene crystals from Brazil [J]. Physica B: Condensed, 2007, 391(2): 322-330.
[20] HOLUJ F. EPR of Mn++ in spodumene. I. Natural crystals [J]. Canadian Journal of Physics, 1968, 46(4): 287-302.
[21] SALIS M. Lattice defects in natural α-spodumene [J]. Il Nuovo Cimento D, 1995, 17(6): 649-651.
[22] SUN Chuan-yao, YIN Wan-zhong. Crystal chemistry analysis on flotability difference of spodumenes with different colors [J]. Nonferrous Metals, 2000, 52(4): 107-110. (in Chinese)
[23] CHEN Ye, CHEN Jian-hua, GUO Jin. A DFT study on the effect of lattice impurities on the electronic structures and floatability of sphalerite [J]. Minerals Engineering, 2010, 14: 1120-1130.
[24] PAYNE M C, TETER M P, ALLAN D C, ARIAS T, JOANNOPOULOS J D. Iterative minimization techniques for ab initio total-energy calculations: molecular dynamics and conjugate gradients [J]. Reviews of Modern Physics, 1992, 64(4): 1045-1097.
[25] PERDEW, J P, WANG Yue. Accurate and simple analytic representation of the electron-gas correlation energy [J]. Physical Review B, 1992, 45(23): 13244-13249.
[26] VANDERBILT D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism [J]. Physical Review B, 1990, 41(11): 892-7895.
[27] LIU Wei-ping, WANG Xu-ming, XU Hui, MILLER J D. Lauryl phosphate adsorption in the flotation of bastnaesite, (Ce,La)FCO3 [J]. Journal of Colloid and Interface Science, 2017, 490: 825-833.
[28] DANA E S, DANA J D. A text-book of mineralogy: With an extended treatise on crystallography and physical mineralogy [M]. New York: Wiley, 1883: 191-203.
[29] PRINCE K C, MATTEUCCI M, KUEPPER K, CHIUZBAIAN S G, BARTKOWSKI S, NEUMANN M. Core-level spectroscopic study of FeO and FeS2 [J]. Physical Review B, 2005, 71(8): 085102.
[30] XU Long-hua, HU Yue-hua, WU Hou-qin, TIAN Jia, LIU Jing, GAO Zhi-yong. Surface crystal chemistry of spodumene with different size fractions and implications for flotation [J]. Separation and Purification, 2016, 169: 33-42.
[31] XU Long-hua, PENG Tie-feng, TIAN Jia, LU Zhong-yuan, HU Yue-hua, SUN Wei. Anisotropic surface physicochemical properties of spodumene and albite crystals: Implications for flotation separation [J]. Applied Surface Science, 2017, 426: 1005-1022.
[32] XU Long-hua, TIAN Jia, DONG Fa-qin, WU Hou-qin, WANG Zhen, WANG Jin-ming. Surface crystal chemistry and anisotropy of spodumene flotation with sodium oleate [J]. The Chinese Jounal of Nonferrous Metals, 2016, 26(10): 2214-2221. (in Chinese)
[33] LIU Cheng, ZHU Guang-li, SONG Shao-xian, LI Hong-qiang. Flotation separation of smithsonite from quartz using calcium lignosulphonate as a depressant and sodium oleate as a collector [J]. Minerals Engineering, 2019, 131: 385-391.
[34] LIU Cheng, ZHANG Wen-cai, SONG Shao-xian, LI Hong-qiang. A novel method to improve carboxymethyl cellulose performance in the flotation of talc [J]. Minerals Engineering, 2019, 131: 23-27.
[35] MULLIKEN R S. Electronic population analysis on LCAO‐MO molecular wave functions. III. Effects of hybridization on overlap and gross AO populations [J]. The Journal of Chemical Physics, 1955, 23(10): 1833-1840.
[36] SEGALL, M D, SHAH R, PICKARD C J, PAYNE M C. Population analysis of plane-wave electronic structure calculations of bulk materials [J]. Physical Review B, 1996, 54(23): 317-320.
[37] SEGALL M D, PICKARD C J, SHAH R, PAYNE M C. Population analysis in plane wave electronic structure calculations [J]. Molecular Physics, 1996, 89(2): 571-577.
朱广丽1,2,王毓华1,王旭明2,Jan D. MILLER2,卢东方1,郑霞裕1,赵悦豪1,郑海涛1
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. College of Mines and Earth Sciences, University of Utah, Salt Lake City 84112, USA
摘 要:研究油酸钠体系中磨矿对锂辉石浮选行为的影响。不同磨矿环境导致锂辉石表面暴露的金属活性位点的含量有差异,从而显著影响锂辉石的可浮性。酸处理后金属活性位点脱离锂辉石表面,使得锂辉石的可浮性下降,进一步表明金属活性位点对锂辉石浮选的重要作用。金属离子杂质可能来源于磨矿环境或晶格杂质。密度泛函理论(DFT)计算表明,杂质铁、钙质点主要是以晶格取代的形式存在于锂辉石表面,镁难以发生晶格取代。与钙和镁相比,铁更易于与油酸钠发生相互作用。扫描电镜结果表明,锂辉石的粒度和解理特性不同,导致其暴露晶面的差异。在不同磨矿环境下,粒度、解理特性和金属离子杂质对油酸钠捕收剂浮选锂辉石起着重要作用。
关键词:磨矿;晶格取代;金属杂质;DFT 计算;锂辉石;解理;浮选
(Edited by Xiang-qun LI)
Foundation item: Project (51674290) supported by the National Natural Science Foundation of China; Project (201606370130) supported by the China Scholarship Council; Project (2016zzts107) supported by the Fundamental Research Funds for the Central Universities of Central South University, China
Corresponding author: Yu-hua WANG, Tel: +86-731-88830545, E-mail: wangyh@csu.edu.cn;
Xu-ming WANG, Tel: +1-801-5851797, E-mail: x.wang@utah.edu
DOI: 10.1016/S1003-6326(19)65060-0

