氧化亚铁硫杆菌浸出废旧锂离子电池中钴酸锂的电化学行为
邓孝荣1,曾桂生1, 2,罗胜联1, 2,罗旭彪1,邹建平1
(1. 南昌航空大学 江西省生态诊断修复与污染阻断重点实验室,江西 南昌,330063;
2. 湖南大学 化学化工学院,湖南 长沙,410082)
摘要:采用自制的粉末微电极,运用开路电位法、循环伏安法、线性扫描伏安法和Tafel法,通过有无氧化亚铁硫杆菌作用来对比研究钴酸锂细菌浸出过程的电化学行为。实验结果表明:在细菌的作用下,钴酸锂在较低电位条件下就能被氧化,在溶液中的腐蚀点电位为0.420 V、致钝电位为0.776 V、钝化电位为0.802 V。无菌条件下,由于钴酸锂氧化电流小,不产生钝化膜。钴酸锂细菌浸出阳极氧化过程的反应具有不可逆性,且反应速度受吸附电化学反应及扩散步骤混合控制。细菌在无菌和有菌条件下的氧化速率分别为 1.544和 1.634 μA/cm2,细菌的加入使电子在钴酸锂电极、溶液界面之间的迁移阻力减小。
关键词:废旧锂离子电池;钴酸锂;生物浸出;电化学
中图分类号:TF19;X705 文献标志码:A 文章编号:1672-7207(2012)07-2500-06
Electrochemical behavior of bioleaching LiCoO2 from spent lithium-ion batteries by Thiobacillus ferrooxidans
DENG Xiao-rong1, ZENG Gui-sheng1, 2, LUO Sheng-lian1, 2, LUO Xu-biao1, ZOU Jian-ping1
(1. Key Laboratory of Ecological Diagnosis-Remediation and Pollution Control of Jiangxi Province,
Nanchang Hangkong University, Nanchang 330063, China;
2. College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, China)
Abstract: Electrochemical behavior on bioleaching of LiCoO2 with a self-produced powder microelectrode in the presence or absence of Thiobacillus ferrooxidans (T. ferrooxidans) was investigated. The methods of open circuit, cyclic voltammetry, linear sweep voltammetry and Tafel methods were used. Experimental results show that LiCoO2 is dissolved at a lower potential in the presence of T. ferrooxidans, and the pitting potential, initiating passive potential and passive potential in the solution are 0.420, 0.776 and 0.802 V, respectively. However, in the absence of T. ferrooxidans, the passive film would not be generated due to the low oxidize current. During the process of anodic oxidation, the oxidation reaction is irreversible, and the oxidation rates are controlled by adsorption and diffusion. The oxidation rates are 1.544 and 1.634 μA/cm2 in the absence and presence of bacteria, respectively, and the electron migratory resistance decreases between the interfaces of electrode/solution with the addition of T. ferrooxidans.
Key words: spent lithium-ion batteries; LiCoO2; bioleaching; electrochemical
锂离子电池具有质量轻、体积小、电压高等优点,成为手机、手提电脑和数码相机等的主要电源[1]。锂离子电池包含很多重金属材料,比如:钴、锂、镉和锰等,这些金属对人类健康和环境具有很大的威胁[2-3]。回收废旧锂电池不仅对保护环境有益,并且能回收利用其中的贵重稀有金属,缓解资源紧缺,使稀有资源得以重复循环利用[4]。目前,对于锂离子电池的回收大多数采用酸浸的方法,可简单有效地浸出锂离子电池中的正极材料钴酸锂。但由于酸浸带来的环境污染以及处理成本较高,可借鉴生物浸出的方法浸出钴酸锂[5-6]。生物浸出具有耗酸量少、处理成本低、常温常压下操作等优点而表现出极好的应用前景,但同时也存在周期长、效率低等问题。辛宝平等[7-8]采用生物浸出废弃锂离子电池中的钴和锂离子,Mishra等[9]采用氧化亚铁硫杆菌浸出废弃锂离子电池中的钴和锂。但仍未见有关浸出机理方面研究的报道。已有研究如应用电化学技术研究一些低品位的硫化矿的浸出过 程[10-12];Li等[13]运用粉末微电极研究硫化矿的细菌浸出过程的氧化过程以及浸出阳极过程动力学;Mu?oz等[14-16]研究块状硫化矿电极的浸出条件和浸出行为。本文运用电化学手段研究氧化亚铁硫杆菌氧化钴酸锂的电化学行为,以便对反应控制步骤及时做出有效的强化措施,为强化钴和锂浸出率提供理论基础。
1 实验方法
1.1 自制钴酸锂粉末微电极
工作电极的制作:剪取1根长4 cm直径为50 μm左右的铂丝和1根长10 cm直径为1 mm左右的铜丝,用直径为50 μm左右的铜丝将他们的两端缠绕联接在一起,使铂丝的露出长度约1.5 cm,将之放于一管壁较厚口径较小的玻璃管中,置于酒精喷灯下高温灼烧,待加热到玻璃管熔点时,用老虎钳对玻璃管进行反复加压,使铂丝紧密封焊于玻璃管端口中,另一端用环氧树脂进行固定。
用水相砂纸、3#和6#金相砂纸依次对电极进行打磨,使铂丝露出玻璃端口表面,并用粉末粒径为0.03 μm的氧化铝对其表面进行抛光。然后分别用自来水冲洗,二次蒸馏水、丙酮超声清洗。将该微电极在微沸的王水中腐蚀铂微盘电极端面。腐蚀过程中用显微镜观察电极,控制腐蚀深度约为20~50 μm。将腐蚀好的电极依次用蒸馏水、丙酮及二次蒸馏水进行超声清洗,待干燥后,再将钴酸锂粉末在玻璃板上经过充分研磨后严实地填人到电极顶端的微孔中,即得到钴酸锂粉末微电极(见图1)。
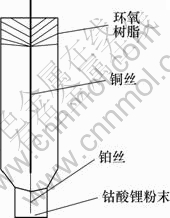
图1 钴酸锂粉末微电极
Fig.1 Powder microelectrode of LiCoO2
1.2 电化学测量系统
电化学测试采用传统的三电极系统,如图2所示。工作电极为钴酸锂粉末微电极,参比电极为饱和甘汞电极,对电极为铂电极。电化学测量仪器为上海振华chi660d电化学工作站。
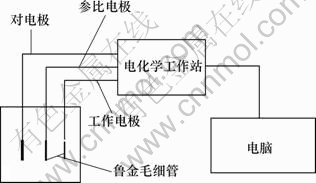
图2 电化学测量系统示意图
Fig.2 Diagram of electrochemical measure system
1.3 溶液及菌种
电化学测量的介质为9K培养基(g/L):各成分的质量百分比分别为(NH4)2SO4 3.0,KCI 0.1,K2HPO4 0.5,MgSO4·7H2O 0.5,Ca(N03)2 0.01,Fe2(SO4)3 48.5,用50%的硫酸调节初始pH值为2.0,菌种为氧化亚铁硫杆菌。实验前预先用9K培养基在30 ℃、150 r/min条件下培养,达到指数生长期时,停止培养并接种(接种量为10%)到电解液中,以保证实验菌种有足够的活性,每次试验后将电极在超声波清洗器中清洗干净。
2 实验结果与讨论
2.1 钴酸锂在9K培养基中的点腐蚀电位
图3所示为钴酸锂粉末微电极分别在无菌、有菌条件下的培养液开路电位。由图3可见:无菌条件下的开路电位明显大于有菌,无菌条件下的开路电位在0.34 V左右,而有菌0.32 V左右。无菌条件下开路电压较高,说明有菌条件下钴酸锂开始氧化腐蚀的电位更低,使得钴酸锂更容易被氧化腐蚀,所以细菌促进钴酸锂的氧化腐蚀。
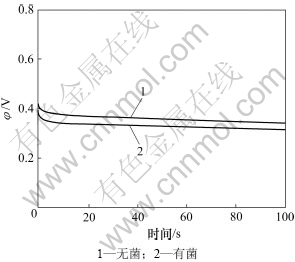
图3 钴酸锂粉末微电极在9K培养基中的开路电位
Fig.3 Open circuit potentials of LiCoO2 powder microelectrodein 9K medium (pH=2, T=298 K)
2.2 钴酸锂浸出的氧化还原过程
2.2.1 无菌钴酸锂电极重复循环伏安
图4所示为钴酸锂粉末微电极无菌条件下的重复循环伏安扫描结果(pH为2,扫描速度Rs=50 mV/s,T=298 K)。由图4可见:在初始阳极方向第1次扫描时,电流在0.581 V后随着电位的增加而明显增加,在1.172 V出现阳极峰,峰电流为6.004 μA,峰电流越大,说明通过粉末微电极的电流越大,极化反应速度较快;阳极氧化后第2次阴极循环时,相同电位区能看见较大的电流峰,此峰电流比初始阳极扫描产生的峰电流强,且随扫描次数增加略增强,表明阳极氧化对阴极还原峰的形成具有重要作用,第2次循环氧化反应程度加强,氧化速率加快。
氧化峰A的反应峰比较宽,可能对应着几个反应,分别为:
2LiCoO2+3H2O→2Co(OH)2+2LiOH+1/2O2+2e,
φ0 (vs. SCE)=0.632 V
Fe2+→Fe3++e, φ0 (vs. SCE)=0.777 V
由实验结果可知:钴酸锂阳极氧化主要是发生钴酸锂水解释放氧气,重复循环扫描时,阳极峰A电位发生移动,说明不是直接的阳极氧化反应电流峰,而是几个反应的叠加。
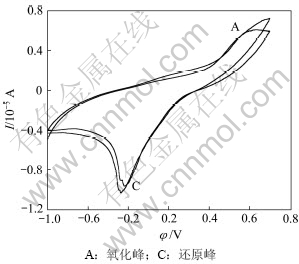
图4 钴酸锂粉末微电极在无菌条件下的重复循环伏安曲线
Fig.4 Repeated cyclic voltammorgans of LiCoO2 powder microelectrode in sterile solution (pH=2, Rs=50 mV/s, T=298 K)
电极负方向往回扫描时,出现了2个紧挨着的明显的还原峰,还原峰C很宽,对应着一系列的还原反应,其中可能发生的反应为:
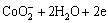 →Co(OH)2+2OH-,
→Co(OH)2+2OH-,
φ0 (vs. SCE)=0.220 V
Fe3++H2O→Fe(OH)3+H+
Fe(OH)3+e→Fe(OH)2+H2O, φ0 (vs. SCE)=-0.560 V
H2O+O2+2e→ +OH-, φ0 (vs. SCE)=-0.076 V
+OH-, φ0 (vs. SCE)=-0.076 V
 +H2O+e→OH+OH-, φ0 (vs. SCE)=-0.245 V
+H2O+e→OH+OH-, φ0 (vs. SCE)=-0.245 V
O2+2H2O+4e→4OH-, φ0 (vs. SCE)=0.401 V
总反应方程式为:
2LiCoO2+H2SO4→2CoSO4+2LiSO4+1/2O2+ 3H2O
2.2.2 有菌钴酸锂电极重复循环伏安
图5所示为钴酸锂电极在有细菌浸出条件下的循环伏安曲线。由图5可见:第1次阳极方向扫描时,有菌和无菌条件下都在0.581 V开始电流随着电位的增加而明显增加,在1.172 V左右出现阳极峰A,但峰电流为8.554 μA,明显大于无菌条件下的峰电流,说明细菌存在时明显加速了亚铁离子的氧化以及钴酸锂的溶解。并且第二次扫描时,在1.134 V电位处峰电流为9.781 μA,此峰电流比第一次扫描产生的峰电流强,且随扫描次数增加略增强,表明阳极氧化对阴极还原峰的形成具有重要作用。
有菌和无菌的区别在于亚铁离子在细菌作用下能更快速的氧化成铁离子:
Fe2++ 2H++1/2O2→Fe3++ H2O +e,
φ0 (vs. SCE)=1.230 V
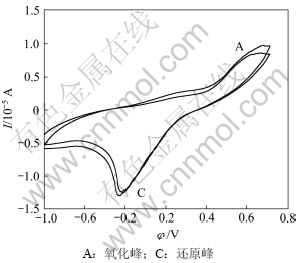
图5 钴酸锂粉末微电极在有菌条件下的重复循环伏安曲线
Fig.5 Repeated cyclic voltammograms of LiCoO2 powder microelectrode with T.ferrooxidan (pH=2, Rs=50 mV/s, T=298 k)
2.3 LiCoO2细菌浸出阳极氧化过程动力学
2.3.1 LiCoO2阳极氧化曲线
图6所示为钴酸锂粉末微电极在温度25 ℃、扫描速度10 mV/s条件下的线性扫描曲线。由图6可知:起始电位在平衡电位附近,当电位向正方向扫描时,电极得电子的还原反应速度将不断增大,电流也不断增大,反应物消耗的量也不断增大,随着电化学反应速率的不断增大,电极表面附近的反应物浓度不断下降,这时反应物由溶液内部向电极表面扩散量的不断增加。当扫描到达0.420 V左右,电流随电位的升高而增大,钴酸锂氧化溶解速度加快,有菌条件下对应着的反应电流明显大于无菌条件下的反应电流。钴酸锂在有菌和无菌酸性体系中的点蚀电位φcorr相同,即φcorr, 1=φcorr, 2=0.420 V,对应着反应电流I2>I1。在有菌条件下,由氧化曲线2可见:当电极正向扫描到0.776 V时,电流迅速减小,形成了一层钝化膜,当电位达到了钝化电位0.802 V以后,电流随扫描电位增加而增加,钝化膜被击穿。所以,由结果可知:在25 ℃、扫描速度10 mV/s条件下,钴酸锂在溶液中的腐蚀电位为0.420 V,致钝电位为0.776 V,钝化电位为0.802 V。在无菌条件下,由于氧化电流小,不产生钝化膜。
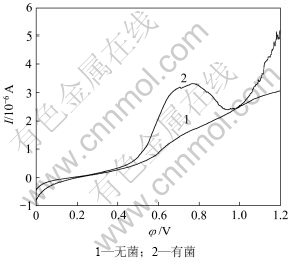
图6 钴酸锂粉末微电极在有无细菌条件下的阳极氧化曲线
Fig.6 Anodic polarization curves of LiCoO2 powder microelectrode in absence or presence of T.ferrooxidan (T=298 K, Rs=10 mV/s)
2.3.2 不同扫描速率LiCoO2细菌浸出阳极极化曲线
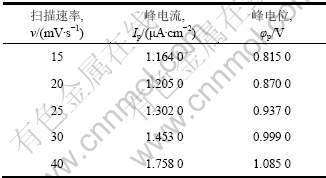
图7所示是钴酸锂电极在有菌9K溶液中不同扫描速度下的线性扫描阳极氧化曲线(温度25 ℃、pH=2.0),由图7中不同扫描速率下的阳极极化曲线可以得出不同扫描速率下对应的峰电流和峰电位,如表1所示。
由表中的峰电流、峰电位分别作出曲线,氧化峰电位和扫描速率的关系可以表示为:
φ=0.281 2 ln v + 0.040 6,回归系数R2 = 0.989 7
氧化峰电流和扫描速率的关系可以表示为:
I=0.000 6v2-0.007 1v+1.132 9,回归系数R2=0.996 6
由结果可知:氧化峰峰电流(IP)既不与扫描速度(v) 的平方根成正比也不与扫描速度成正比,氧化峰峰电位与ln v成正比关系,且向扫描速率增加的方向移动。可以推断,钴酸锂细菌浸出阳极氧化过程的反应具有不可逆性,且反应速度受吸附电化学反应及扩散步骤混合控制。
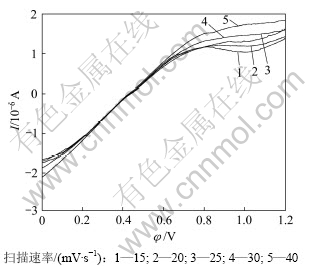
图7 不同扫描速率下钴酸锂阳极氧化曲线
Fig.7 Anodic polarization cuvres of LiCoO2 oxidation by T.ferrooxidan under different scanning rates(T=298 K, Rs=15~40 mV/s)
表1 阳极氧化曲线结果
Table 1 Results of anodic polarization curves
2.3.3 有无细菌Tafel曲线
图8所示为有、无细菌条件下,钴酸锂粉末微电极在温度25 ℃、扫描速度0.01 mV/s的Tafel曲线,以电流电位作φ-I线性极化曲线,采用最小二乘法线性回归方法求出最佳直线方程,其直线的斜率即为钴酸锂电极的极化阻力Rp,而直线的截距近似为钴酸锂电极的腐蚀电位φcorr。阴极和阳极过程的Tafel常数ba和bc分别对应着?φ-logI极化曲线直线部分的斜 率。钴酸锂电极的腐蚀线性极化方程符合Stern-Geary方程:
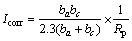
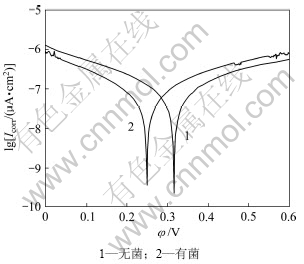
图8 钴酸锂粉末微电极在有、无细菌条件下的Tafel曲线
Fig.8 Tafel cuvres of LiCoO2 powder microelectrode in the presence and absence of T.ferrooxidans
(pH=2, T=298 K, Rs=10 mV/s)
由图8的Tafel曲线计算得出相关数据如表2所示。由表2可知:在有细菌的条件下,腐蚀电流密度增大、极化阻力降低,这表明钴酸锂氧化反应速率有所提高,并且加入细菌后,阴极Tafel斜率bc=2.3RT/αnF增加,阳极Tafel斜率ba=2.3RT/βnF减少。如果电子转移数n相等,则电子转移系数α减小,β增加。所以由结果可知:细菌的加入有利于钴酸锂阳极反应的进行,抑制阴极反应的进行;而且电极表面沉积的钝化膜又出现溶解现象,使得电子在钴酸锂电极、溶液界面之间的迁移阻力减小,α减小,β增大。体系的腐蚀电位负移、腐蚀电流密度增大、极化(或迁移)阻力降低,细菌促进阳极反应。
表2 钴酸锂细菌浸出的动力学参数
Table 2 Bioleaching kinetic parameter of LiCoO2

3 结论
(1) 通过电化学方法,探明钴酸锂细菌浸出的中间过程为:亚铁离子在细菌的作用下氧化为铁离子,铁离子通过水解产生H+,并且还原成亚铁离子,产生H+能溶解浸出钴酸锂,所以在加入钴酸锂之后促进铁离子的还原反应的进行,这也解释为什么在没有加入钴酸锂之前溶液是棕红色,加入钴酸锂颜色明显的变淡,变为淡黄色的原因。
(2) 由钴酸锂的浸出的动力学参数可知,钴酸锂在0.220 V左右发生阴极还原反应,在0.632 V左右发生钴酸锂阳极溶解,所以在浸出体系中加以外控电位,可以加速钴酸锂的溶解。
(3) 钴酸锂阳极溶解反应具有不可逆性,且反应速率受吸附和扩散反应混合控制。
(4) 细菌的加入有利于钴酸锂阳极反应的进行,抑制阴极的进行,使电子在钴酸锂电极、溶液界面之间的迁移阻力减小,加快钴酸锂的氧化速率。
参考文献:
[1] Jessica F P, Natalia G B, Julio C A, et al. Recovery of valuable elements from spent Li-batteries[J]. Journal of Hazardous Materials, 2008, 150(3): 843-849.
[2] Hal A, Angelica V S. Toxicity of lithium to humans and the environment—A literature review[J]. Ecotoxicology and Environmental Safety, 2008, 70(3): 349-356.
[3] Castillo S, Ansart F, Laberty-Robert C, et al. Advances in the recovering of spent lithium battery compounds[J]. Journal of Power Sources, 2002, 112(1): 247-254.
[4] Paulino J F, Busnardo N G, Afonso J C, et al. Recovery of valuable elements from spent Li-batteries[J]. Journal of Hazardous Materials, 2008, 150(3): 843-849.
[5] LI Li, GE Jing, WU Feng. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. Journal of Hazardous Materials, 2010, 176(1/2/3): 288-293.
[6] XU Jin-qiu, Thomas H R, Francis R W, et al. A review of processes and technologies for the recycling of lithium-ion secondary batteries[J]. Journal of Power Sources, 2008, 177(2): 512-527.
[7] 辛宝平, 朱庆荣, 李是珅, 等. 生物淋滤溶出废旧锂离子电池中钴的研究[J]. 北京理工大学学报, 2007, 27(6): 551-555.
XIN Bao-ping, ZHU Qing-rong, LI Shi-kun, et al. Study on the release of Co from retrieved Li-ion batteries by bioleaching[J]. Transactions of Beijing Institute of Technology, 2007, 27(6): 551-555.
[8] XIN Bao-ping, ZHANG Di, ZHANG Xian, et al. Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria[J]. Bioresource Technology, 2009, 100(24): 6163-6169.
[9] Debaraj M, Kim D J, Ralph D E, et al. Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans[J]. Waste Management, 2008, 28(2): 333-338.
[10] Bevilaqua D, Acciari H A, Benedetti A V, et al. Electrochemical noise analysis of bioleaching of bornite (Cu5FeS4) by Acidithiobacillus ferrooxidans[J]. Hydrometallurgy, 2006, 83(1/2/3/4): 50-54.
[11] Lee K Y, Yoon I H, Lee B T, et al. A novel combination of anaerobic bioleaching and electrokinetics for arsenic removal from mine tailing soil[J]. Environmental Science & Technology, 2009, 43(24): 9354-9360.
[12] Zohreh S, Amir L, Amir F, et al. Possibility of using chemical fertilizers instead of 9K medium in bioleaching process of low-grade sul?de copper ores[J]. Hydrometallurgy, 2009, 96(3): 264-267.
[13] LI Hong-xu, QIU Guan-zhou, HU Yue-hua, et al. Electrochemical behavior of chalcopyrite in presence of Thiobacillus ferrooxidans[J]. Transactions of Nonferrous Metals Society of China, 2006, 16(5): 1240-1245.
[14] Mu?oz J A, Blázquez M L, González F, et al. Electrochemical study of enargite bioleaching by mesophilic and thermophilic microorganisms[J]. Hydrometallurgy, 2006, 84(3/4): 175-186.
[15] Olubambi P A, Potgieter J H, Ndlovu S, et al. Electrochemical studies on interplay of mineralogical variation and particle size on bioleaching low grade complex sulphide ores[J]. Transactions of Nonferrous Metals Society of China, 2009, 19(5): 1312-1325.
[16] Hansford G S,Vargas T. Chemical and electrochemical basis of bioleaching processes[J]. Hydrometallurgy, 2001, 9(1): 13-26.
(编辑 邓履翔)
收稿日期:2011-04-29;修回日期:2011-06-21
基金项目:江西省自然科学基金项目(2010GZH0111);江西省教育厅青年科学基金项目(GJJ11167)
通信作者:曾桂生(1977-),男,江西宁都人,副教授,博士,从事电子废弃物处理及资源化研究;电话:0791-83953377;E-mail: zengguisheng@hotmail.com