砷化氢析出电势的探讨
来源期刊:中国有色金属学报2000年第1期
论文作者:仇勇海 唐仁衡 陈白珍
文章页码:101 - 104
关键词:砷化氢;析出电势;铜电解液
Key words:arsine; evolution potential; copper electrolyte
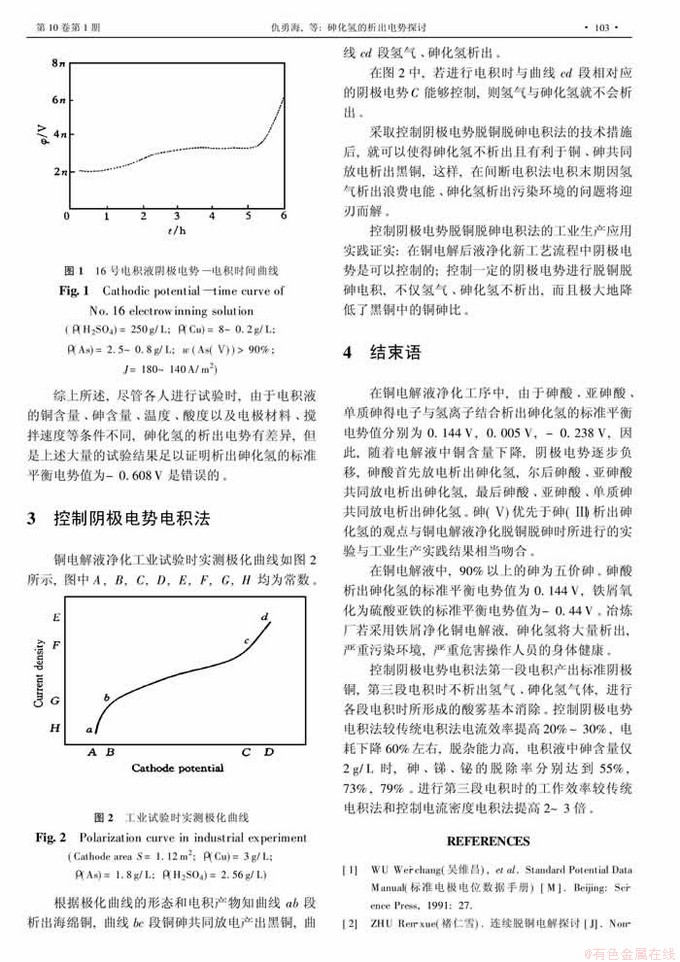
摘 要:据热力学参数,计算得到砷酸、亚砷酸、单质砷析出砷化氢的标准平衡电势值分别为0.144 V, 0.005V,-0.238 V。在铜电解液净化工序中,砷酸优先于亚砷酸析出砷化氢,当阴极电势足够负时,三者共同放电析出砷化氢。计算结果与实测结果基本吻合,均表明砷化氢析出的标准平衡电势值正于-0.608 V。在铜净液工序中,采用控制阴极电势脱铜脱砷电积法,能有效抑制砷化氢的析出。
Abstract: The standard equilibrium potentials of evolving AsH3 from arsenic acid, arsenious acid and element arsenic are thermodynamically calculated as 0.144V, 0.005V, -0.238 V respectively. In the processing of the purification of copper electrolyte, arsenic acid prior to arsenious acid evolves AsH3. When cathodic potential is negative enough, arsenic acid, arsenious acid and element arsenic co-discharge and AsH3 is evolved. The results of thermodynamical calculation are basically concordance with the measured results. The standard equilibrium potential of AsH3 evolution is absolutely more positive than -0.608V. In the processing of the purification of copper electrolyte, the separation of copper and arsenic by electrowinning of controlling cathodic potential can prevent AsH3 from evolving effectively.