High efficient Sr/S isolation for preparing Sr(OH)2 from celestite (SrSO4) in alkaline solution
来源期刊:中南大学学报(英文版)2019年第1期
论文作者:段东平 陈思明 刘艳 周娥 韩宏亮 邹兴武
文章页码:219 - 228
Key words:celestite; Sr(OH)2; high efficient Sr/S isolation; dissolution-precipitation process
Abstract: The bottleneck of strontium compounds preparing from celestite is the promotion of Sr/S isolation efficiency. Low energy consumption and zero release method for isolating Sr/S in preparing Sr(OH)2 process from celestite in mild condition was described. Sr element remained in precipitation with formation of Sr(OH)2, while S element entered into leachate with formation of Na2SO4. The effects of initial concentration of NaOH, conversion temperature, liquid-to-solid (L/S) ratio and conversion time on Sr/S ratio of samples for celestite conversion were systematically investigated by experiments. The results demonstrated that the efficiency of Sr/S isolation increased with the initial concentration of NaOH, L/S ratio and conversion time, and decreased with conversion temperature. The maximum conversion ratio of Sr(OH)2 was 93.88% under the optimum condition, whose Sr/S ratio of sample could reach to 41.16. It illustrated that better isolation efficiency of celestite could be achieved in alkaline treatment. The results of SEM-EDS analyses demonstrated that the conversion reaction was a dissolution-precipitation process.
Cite this article as: CHEN Si-ming, DUAN Dong-ping, LIU Yan, ZHOU E, HAN Hong-liang, ZOU Xing-wu. High efficient Sr/S isolation for preparing Sr(OH)2 from celestite (SrSO4) in alkaline solution [J]. Journal of Central South University, 2019, 26(1): 219–228. DOI: https://doi.org/10.1007/s11771-019-3995-9.

J. Cent. South Univ. (2019) 26: 219-228
DOI: https://doi.org/10.1007/s11771-019-3995-9
CHEN Si-ming(陈思明)1, 2, 3, DUAN Dong-ping(段东平)4, LIU Yan(刘艳)4,ZHOU E(周娥)4, HAN Hong-liang(韩宏亮)4, ZOU Xing-wu(邹兴武)1, 2, 3
1. Key Laboratory of Comprehensive and Highly Efficient Utilization of Salt Lake Resources,Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xi’ning 810008, China;
2. Qinghai Engineering and Technology Research Center of Comprehensive Utilization of Salt Lake Resources, Key Laboratory of Salt Lake Resources Chemistry of Qinghai Province, Xi’ning 810008, China;
3. University of Chinese Academy of Sciences, Beijing 100049, China;
4. Key Laboratory of Green Process Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: The bottleneck of strontium compounds preparing from celestite is the promotion of Sr/S isolation efficiency. Low energy consumption and zero release method for isolating Sr/S in preparing Sr(OH)2 process from celestite in mild condition was described. Sr element remained in precipitation with formation of Sr(OH)2, while S element entered into leachate with formation of Na2SO4. The effects of initial concentration of NaOH, conversion temperature, liquid-to-solid (L/S) ratio and conversion time on Sr/S ratio of samples for celestite conversion were systematically investigated by experiments. The results demonstrated that the efficiency of Sr/S isolation increased with the initial concentration of NaOH, L/S ratio and conversion time, and decreased with conversion temperature. The maximum conversion ratio of Sr(OH)2 was 93.88% under the optimum condition, whose Sr/S ratio of sample could reach to 41.16. It illustrated that better isolation efficiency of celestite could be achieved in alkaline treatment. The results of SEM-EDS analyses demonstrated that the conversion reaction was a dissolution-precipitation process.
Key words: celestite; Sr(OH)2; high efficient Sr/S isolation; dissolution-precipitation process
Cite this article as: CHEN Si-ming, DUAN Dong-ping, LIU Yan, ZHOU E, HAN Hong-liang, ZOU Xing-wu. High efficient Sr/S isolation for preparing Sr(OH)2 from celestite (SrSO4) in alkaline solution [J]. Journal of Central South University, 2019, 26(1): 219–228. DOI: https://doi.org/10.1007/s11771-019-3995-9.
1 Introduction
Celestite (main component is strontium sulfate, SrSO4) is the primary mineral in nature for strontium compounds production [1–3]. As is well-known, SrSO4, which has similar behavior like BaSO4, is an insoluble mineral (Ksp=3.2×10–7,25 °C). Hence, the bottleneck of strontium compounds production in SrSO4 conversion is how to promote Sr/S isolation efficiency. Regarded as the most important intermediate raw material, strontium carbonate (SrCO3) has the largest output in strontium plants. Researchers find that SrSO4 could be reduced to strontium sulfide (SrS) at high temperature. As a result, a method for strontium carbonate production called black ash process is carried out as follows [4]: celestite roasts with coke to prepare SrS, and then soluble SrS solution is obtained by hot water leaching (80–85 °C). At last, Sr/S isolation is carried out with adding CO2 or carbonate. In this condition, Sr element remains in precipitate with formation of SrCO3, while S element remains in gas or liquid phase. The black ash process is carried out economically in most strontium carbonate plants in the world as its virtues likes simple operation, high product quality and so on. However, high energy consumption and formation of hazardous pollutants restricts the usage. Many attempts have been made for reducing energy consumption [5, 6] or CO2 emission [7–9], but these methods do not fundamentally solve the practical problems. In addition, H2S is an inevitable product in this process as well.
The increasing concerns over CO2 and H2S emissions lead to need of the alternative—the double decomposition process. In this process, the conversion reaction obtaining SrCO3 takes place in solution, where finely powdered celestite reacts with carbonate. In this condition, SO42– ions are substituted by CO32– ions in solution because of the lower solubility of SrCO3 (Ksp=5.6×10–10, 25 °C). Crude SrCO3 is obtained, and the technical grade SrCO3 is obtained by dissolution-recarbonization process of crude SrCO3 at last. The double decomposition process also has its own defects, such as repeated carbonization-acid dissolution– recarbonization operation, high difficult impurity removal process and low stability of product quality which hinders its popularization and wide application. Many researchers have tried to make improvements [10–12], but it still cannot meet the needs of industrial production.
To sum up, direct conversion of celestite for preparing strontium compounds must be one of the best approaches since it has easier operation and controlling of product quality. Many attempts have been made. AYDO AN et al [13] attempt to dissolve the celestite in acidic BaCl2 solution. In that condition, SO42– is precipitated with formation of BaSO4, while Sr2+ from SrSO4 can convert to SrCl2 in solution. SU
AN et al [13] attempt to dissolve the celestite in acidic BaCl2 solution. In that condition, SO42– is precipitated with formation of BaSO4, while Sr2+ from SrSO4 can convert to SrCl2 in solution. SU REZ-ORDUNA et al [14] find that F– ions will exchange SO42– under 250 °C in NaF solutions, for converting celestite into SrF2. REND
REZ-ORDUNA et al [14] find that F– ions will exchange SO42– under 250 °C in NaF solutions, for converting celestite into SrF2. REND N-ANGELES et al [15] treat barite- celestite crystals in highly concentrated alkaline hydrothermal fluid (5 mol/L KOH) coexisting with Ti(OH)4·4.5H2O to produce SrTiO3 particles under 250 °C. In this process, BaSO4 and SrSO4 are dissolved in high concentration alkaline hydrothermal media, and simultaneous dehydration of the Ti-gel. SrTiO3 is synthesized with reaction between Sr(OH)2 and Ti(OH)4. ZORAGA et al [16] find that celestite can convert to acidic strontium oxalate in H2C2O4 solution. In H2C2O4 solution, SrSO4 is firstly converted to SrC2O4·2H2O, and then H[Sr(C2O4)·1.5(H2O)] forms. At last, Sr(HC2O4)(C2O4)0.5·2H2O is obtained from solution.
N-ANGELES et al [15] treat barite- celestite crystals in highly concentrated alkaline hydrothermal fluid (5 mol/L KOH) coexisting with Ti(OH)4·4.5H2O to produce SrTiO3 particles under 250 °C. In this process, BaSO4 and SrSO4 are dissolved in high concentration alkaline hydrothermal media, and simultaneous dehydration of the Ti-gel. SrTiO3 is synthesized with reaction between Sr(OH)2 and Ti(OH)4. ZORAGA et al [16] find that celestite can convert to acidic strontium oxalate in H2C2O4 solution. In H2C2O4 solution, SrSO4 is firstly converted to SrC2O4·2H2O, and then H[Sr(C2O4)·1.5(H2O)] forms. At last, Sr(HC2O4)(C2O4)0.5·2H2O is obtained from solution.
Among many strontium compounds, Sr(OH)2 has a unique property-larger variation of solubility under different temperatures. It is readily purified by re-crystallization process. As a result, Sr(OH)2 is regarded as a substitutional material of SrCO3 for manufacturing high purity strontium compounds. Nowadays, technique is used to preparing Sr(OH)2 from SrCO3. SrCO3 converts to SrO or soluble strontium compounds (e.g., SrCl2 or Sr(NO3)2) by pyrolysis or acid-dissolution, and then the product reacts with water or alkaline to prepare Sr(OH)2. In these processes, excessive energy consumption (carbothermic reduction of SrSO4) and complex processes (e.g., repeated acid-base operation) result in increase of production cost. The effects of alkaline hydroxides on the direct mechanochemical carbonation of SrSO4 under CO2 atmosphere is researched by TURIANICOV et al [17]. In their research, they find that hydroxyl can substitute the sulfate radical in dry high-energy milling process of SrSO4 and NaOH mixture. Sr(OH)2 and Na2SO4 are formed in solid phase at last. It confirms the probability of Sr(OH)2 direct conversion. But the efficiency of Sr/S isolation is still not guaranteed because of hardly separation of Sr(OH)2 from product.
et al [17]. In their research, they find that hydroxyl can substitute the sulfate radical in dry high-energy milling process of SrSO4 and NaOH mixture. Sr(OH)2 and Na2SO4 are formed in solid phase at last. It confirms the probability of Sr(OH)2 direct conversion. But the efficiency of Sr/S isolation is still not guaranteed because of hardly separation of Sr(OH)2 from product.
In this study, an alkaline treatment of celestite was carried out to obtain the high efficiency Sr/S isolation under more mild condition. The entire reaction could be carried out in room temperature (25 °C). In this condition, Sr element was precipitated with the formation of Sr(OH)2, while S element was entered into leachate with the formation of Na2SO4. Effects of various factors on the efficiency of Sr/S isolation were studied, including initial concentration of NaOH, conversion temperature, liquid-to-solid ratio (L/S) and conversion time. The scanning electron microscope (SEM) and energy dispersive spectrometer (EDS) analyses were carried out to investigate the isolation mechanism.
2 Experimental
2.1 Materials preparation
The celestite concentrate (<200 μm) is collected from Dafengshan Mine, Qinghai, China, and pre-washed with dilute HCl solution (pH=1) to remove carbonate impurity. After acid washing and filtration, washing with distilled water were carried out, and then the solid residues were dried at 105 °C overnight. The major constituents of pre-treated celestite were as follows: SrO 47.42 wt%, SO3 39.82 wt%, SiO2 5.81 wt%, which corresponded to SrSO4 83.89 wt% and SiO2 5.81 wt%. The contents of minor elements in minerals were as follows: CaO 1.56 wt%, MgO 0.69 wt%, BaO 1.10 wt% and Fe2O3 0.49 wt%, which corresponded to CaSO4 3.78 wt%, BaSO4 1.68 wt% and MgO 0.69 wt%. Hence the Sr/S ratio of pristine mineral was 2.52. X-ray diffraction powder analysis of the pre-treated celestite was carried out and the result is shown in Figure 1. The principal diffraction peaks of 2θ values at 23.6°, 27.1°, 30.1°, 32.8°, 44.3° and 50.7° corresponded to SrSO4 which belonged to orthorhombic system (JCPDS card No. 01-089- 7355). The diffraction peaks corresponding to CaSO4, BaSO4, MgO and SiO2 were not presented since the contents of them in pre-treated celestite were too low.
NaOH and hydrochloric acid of analytical grade were purchased from Sinopharm Chemical Reagent Co., Ltd. Deionized water prepared by UP water purification system (Ulupure Co.) was used in this experiment.
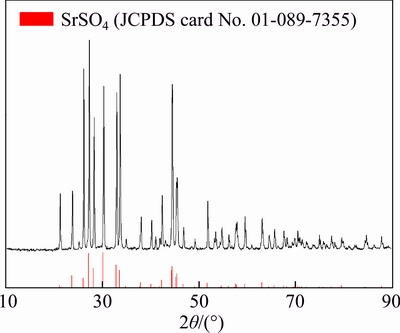
Figure 1 XRD pattern of celestite after pre-treatment
2.2 Conversion procedure
The main reaction of celestite conversion in alkaline solution is assumed to occur as:
SrSO4(s)+2NaOH(aq)=Sr(OH)2(s)+Na2SO4(aq) (1)
Alkaline conversion experiments were performed by mixing the pre-treated celestite and NaOH solution (Eq. (1)) at a predetermined L/S ratio in 200 mL Teflon reactor with nitrogen atmosphere. The reactor was put into a water bath at stable temperature for a certain time. After reaction completed, the reactor cooled to room temperature (ca. 25 °C) rapidly and slurry was filtrated. The solid residue was washed fully with 10 wt% NaOH solution at room temperature (ca. 25 °C), and dried at 40 °C overnight in vacuum oven. The product was analyzed at last.
2.3 Analytical methods
Morphology of samples were characterized by SEM and energy dispersive X-ray spectroscope (EDS) using MLA Quanta 250 (FEI company Co. Ltd). The phases of samples were characterized by X-ray diffractometer (XRD) using B.V. X’pert pro MPD (PANalytical Co. Ltd), with Cu Kα radiation (40 kV and 40 mA), scanning from 2θ=10° to 90° at rate of 15(°)/min. The FT-IR spectra were characterized by Fourier transform infrared spectroscopy using T27-Hyprion-Vector22 (Bruker Co. Ltd), scanning from 400 to 4000 cm–1. Phase compositions of samples were determined by X-ray fluorescence (XRF) using B.V. AXIOS (PANalytical Co. Ltd).
As no Sr content was detected in conversion solutions which were obtained with reaction completed. Sr/S ratio of samples was measured by dissolving with hydrochloric acid solution (pH=2) and determining the compositions of products by XRF after fully washing and drying as well. It was evaluated as follow:
 (2)
(2)
where γSr/S is the Sr/S ratio of sample; ωSr and ωS are the Sr and S element contents of sample determined by XRF analysis (%), respectively.
It is known to all that, carbonate hardly exists in acidic solution (pH<2). Thus, SrCO3 seldom exists in pre-treated celestite after fully washing with hydrochloric acid (pH=2). The conversion ratio of Sr(OH)2 was measured as follow:
 (3)
(3)
where αSr(OH)2 is the conversion ratio of Sr(OH)2 (%); nSr is the content of Sr in sample obtained after alkaline conversion of celestite (mol); MSr is the molar mass of Sr (87.62 kg/mol); mSrSO4is the mass of pristine SrSO4 (kg); and ωSris the mass fraction of Sr in pristine celestite (%).
3 Results and discussion
3.1 Effect of initial concentration of NaOH
Different initial concentrations of NaOH in conversion reaction were carried out to evaluate the feasibility for isolation of Sr and S. The XRD patterns of products obtained at 25 °C for 1 h varying the initial concentration of NaOH are shown in Figure 2. Only the principal diffraction peaks belonging to SrSO4 were observed in the sample obtained under condition of 10 wt% NaOH solution. It implied that few Sr(OH)2·8H2O was generated. New diffraction peaks located at 13.9°, 20.7° and 36.9°, which corresponded to the Sr(OH)2·8H2O (JCPDS card No. 01-073-2138), began to display in the sample by using 15 wt% NaOH solution. It confirmed that a new phase of Sr(OH)2·8H2O was generated in this reaction. The increasing of the initial concentration of NaOH resulted in a progressive increasing content of Sr(OH)2·8H2O in sample as its intensity value of diffraction peaks belonged to Sr(OH)2·8H2O increasing with the concentration of initial NaOH. No diffraction peak belonging to SrCO3 was detected in these samples since nitrogen atmosphere was used in the entire reaction process.

Figure 2 XRD patterns of samples treated by different initial concentrations of NaOH under condition of reaction temperature 25 °C, conversion time 1 h, L/S ratio 10 mL/g
The effects of the initial concentration of NaOH on Sr/S ratio of samples for celestite conversion are shown in Figure 3. Sr/S ratio of sample was used to index the efficiency of isolation of Sr/S. High Sr/S ratio of sample demonstrated that less SO42– remained in strontium compounds. As mentioned above, in pristine material, the Sr/S ratio was 2.52. With the initial concentration of NaOH solution increasing, the Sr/S ratio of sample slowly increased firstly (2.54 of 10 wt% and 2.75 of 15 wt%, respectively), and then sharply increased (from 2.75 of 15 wt.% to 5.41 of 30 wt%). It demonstrated that higher initial concentration of NaOH solution was benefit for conversion reaction. Similar behavior of alkaline leaching process was obtained by  AHIN et al [18]. Further higher initial concentrations of NaOH solution was not discussed since reactor corrosion would be accelerated in actual production by using NaOH solution beyond 30 wt%.
AHIN et al [18]. Further higher initial concentrations of NaOH solution was not discussed since reactor corrosion would be accelerated in actual production by using NaOH solution beyond 30 wt%.
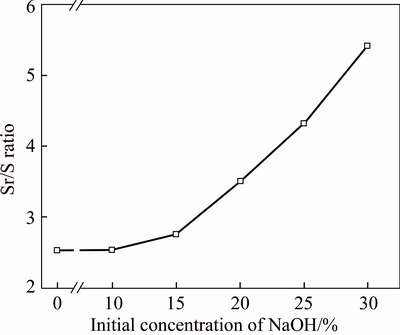
Figure 3 Relationship between initial concentration of NaOH (CNaOH) and Sr/S ratio of samples (γSr/S) under condition of reaction temperature 25 °C, conversion time 1 h, L/S ratio 10 mL/g
of NaOH (CNaOH) and Sr/S ratio of samples (γSr/S) under condition of reaction temperature 25 °C, conversion time 1 h, L/S ratio 10 mL/g
3.2 Effect of conversion temperature
It is known that the solubility of Sr(OH)2 drastically increases with temperature. Therefore, as-prepared Sr(OH)2 will re-dissolve in alkaline solution and regenerated to SrSO4 with SO42– ions at high temperatures. The XRD patterns of the product obtained at different conversion temperatures are shown in Figure 4. It showed that the intensity of diffraction peaks belonging to Sr(OH)2 decreased when temperature promoted. The diffraction peaks belonging to Sr(OH)2 disappeared at 60 °C, confirming that few Sr(OH)2 existed in deposition above 60 °C.
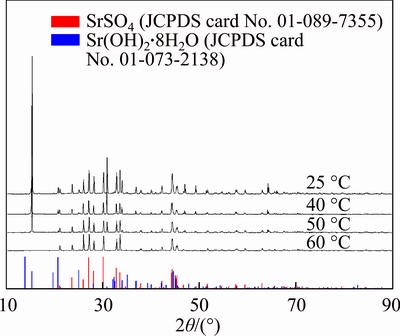
Figure 4 XRD patterns of samples treated by different reaction temperatures under condition of L/S ratio 10 mL/g, conversion time 1 h, initial concentration of NaOH 20 wt% (top to bottom: 25, 40, 50 and 60 °C)
The effects of the conversion temperature on Sr/S ratio of samples for celestite conversion were studied. Figure 5 confirmed that conversion temperature had serious impact on isolation of Sr and S element. The Sr/S ratio of samples decreased rapidly with temperature increase. The data obtained at 60 °C decreased to 2.55, much close to the pristine material (2.52). It revealed that most of Sr(OH)2 has been converted to SrSO4 again at that temperature.

Figure 5 Relationship between reaction temperature (T) and Sr/S ratio of samples (γSr/S) under condition of L/S ratio 10 mL/g, conversion time 1 h, initial concentration of NaOH 20 wt%
3.3 Effect of liquid-to-solid ratio
Liquid-to-solid (L/S) ratio is an index for surface contact. High L/S ratio improves surface contact between alkaline solution and celestite particles, which will increase reaction rate of Sr(OH)2 preparing. The Sr/S ratio of samples obtained at different L/S ratios were studied and shown in Figure 6. Results revealed that at low L/S ratio (5 mL/g), the Sr/S ratio of sample was only 3.03. While the data improved fiercely, reaching to 6.53 at 40 mL/g. Even at 100 mL/g, the value would reach to 262.4. It demonstrated that most of the SrSO4 have converted to Sr(OH)2. A similar behavior was obtained by LIU et al [19] as well. However, in industrial production, excessive pursuit of high conversion ratio by using higher L/S ratio would increase consumption of NaOH and reduce production efficiency. Therefore, an appropriate L/S ratio should be employed with comprehensive consideration.
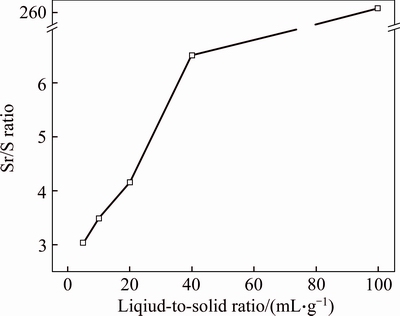
Figure 6 Relationship between liquid-to-solid ratio (L/S ratio) and Sr/S ratio of samples (γSr/S) under condition of reaction temperature 25 °C, conversion time 1 h, initial concentration of NaOH 20 wt%
3.4 Effect of conversion time
The effects of conversion time on Sr/S ratio of samples obtained at different L/S ratio are shown in Figure 7. As mentioned above, the L/S ratio would significantly affect the conversion of Sr(OH)2. Under low S/L ratio as 10 mL/g, the Sr/S ratio of sample only increased from 3.49 for 1 h to 5.35 for 6 h. Raising the L/S ratio can improve the conversion rate of Sr(OH)2 and decrease the concentration of SO42– in solution. Therefore, better efficiency of Sr/S isolation can be achieved during prolonging time at higher L/S ratio. The Sr/S ratio in the sample which was obtained under the conditions of S/L ratio as 40 mL/g and conversion time as 6 h could reach to 41.16. It was almost 6 times the data obtained for 1 h (6.53). The conversion ratio of Sr(OH)2 in this sample would reach 93.88% as well. The sample was dried at 130 °C for 48 h, and its XRD test was carried out. The result of XRD pattern shown in Figure 8 demonstrated that two species as Sr(OH)2 and Ca0.7Sr0.3SO4 can be detected. And the diffraction peaks intensity belonging to Sr(OH)2 were much higher than Ca0.7Sr0.3SO4. It implied that few SrSO4 and CaSO4 were remained in conversion product. The FT-IR test of the sample was also carried out to testify this deduction. The result shown in Figure 9 illustrated that sharp bands at 3609 and 3591 cm–1 were assigned to OH groups. The small band at 3665 cm–1 corresponded to the water. It may be absorbed from air in the pressuring process of the FT-IR testing sample. The broad band at approximately 3479 cm–1 represented characteristic intermolecular hydrogen bonding. The band at 1700 cm–1 was considered to the stretching mode of the OH group (O—H). And the absorption band at 1456 cm–1 corresponded to OH group (O—H) deformation vibration. The bands at 1199, 1130 and 1091 cm–1 corresponded to SO42– group (S—O) asymmetric stretching vibrations, while the bands at 993, and 949 cm–1 corresponded to SO42– group (S—O) bending vibrations. The bands at 653 and 611 cm–1 may represent Sr—O or Ca—O bands in sample [20, 21]. It implied that Sr(OH)2 and sulfate would remain in conversion product. Therefore, all of the results were in good accordance with the deduction that SrSO4 in celestite could efficiently convert to Sr(OH)2 in precipitate under suitable alkaline condition.
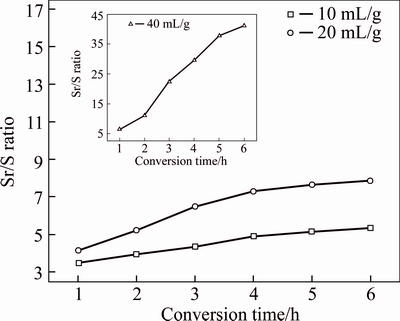
Figure 7 Relationship between conversion time (t) and Sr/S ratio of samples (γSr/S) at different L/S ratio under condition of reaction temperature 25 °C, initial concentration of NaOH 20 wt%

Figure 8 XRD patterns of sample obtained under condition of L/S ratio 10 mL/g, conversion time 1 h, initial concentration of NaOH 20 wt%, temeprature 25 °C and dried at 130 °C for 48 h
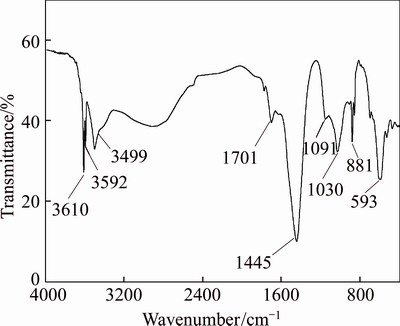
Figure 9 FT-IR spectrum of sample obtained under condition of L/S ratio 10 mL/g, conversion time 1 h, initial concentration of NaOH 20 wt%, temperature 25 °C and dried at 130 °C for 48 h
As mentioned above, acid leaching and recarbonization process is used to separate SrCO3 from unreacted SrSO4 in the double decomposition process. The repeated carbonization-acid dissolution-recarbonization procedure causes the increasing of cost for preparing SrCO3. And number of impurities are entered into leachate in acid dissolution process. As a result, it increases the difficulty of purification before recarbonization process. Furthermore, prepared SrCO3 should convert to soluble strontium compounds, e.g., Sr(OH)2 before synthesis of high-purity strontium compound. The newly prepared Sr(OH)2 in conversion product of celestite obtained though alkaline treatment could be extracted by hot water leaching and crystallization process. And it could be directly used in purification process as well. Obviously, the conversion treatment for preparing Sr(OH)2 from celestite in alkaline solution is superior to that in double decomposition process. There is no further discussion on extraction process of Sr(OH)2 in this paper since the extraction process has been widely applied in industrial production for purification of Sr(OH)2.
3.5 Mechanism of conversion
Dissolution behavior would occur with the introduction of SrSO4 into an aqueous solution. Sr2+
and SO42– ions in solution were formatted depending on the reaction pH [22]. The equilibrium reactions with the solubility products (lgKsp)(25 °C) of SrSO4 were shown as follows:
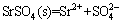 , lgKsp= –6.62 (4)
, lgKsp= –6.62 (4)
 , lgK= –2.29 (5)
, lgK= –2.29 (5)
Moreover, the solubility product (lg Ksp)(25 °C) of Sr(OH)2 was shown as follow [23]:
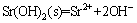 , lgKsp= –28.45 (6)
, lgKsp= –28.45 (6)
Therefore, it implied that, in suitable concentration of alkaline solution, Sr2+ ions, which were dissolved in solution, could be deposited with formation of Sr(OH)2. The substantial decrease of Sr2+ ions in alkaline solution urged to have SO42– ions released out of SrSO4. Therefore, Sr element from SrSO4 would be remained in precipitation with formation of Sr(OH)2, while S element entered into alkaline solution with formation of Na2SO4. And the isolation of Sr and S elements would be realized in the conversion process.
The morphology of treated samples for different operation conditions were characterized by SEM analysis, and the compositions of samples were measured by EDS. The results are shown in Figure 10 and Table 1. Typical morphology of pristine celestite ore with compacted solid phases on the surface was found in Figures 10(a) and (b). At the intermediate stage of reaction (1 h), a significant amount of tiny cylinder crystal covered with the surface of celestite (shown in Figures 10(c) and (d)). When the reaction was carried out for a prolonging interval (6 h), cylindrical precipitates which were quite different with the starting material have been discovered (shown in Figures 10(e) and (f)). The EDS data, obtained from Figure 10(e) and listed in Table 1 demonstrated that no S content was detected in the crystal, which confirmed that good efficiency of Sr/S isolation was guaranteed.
The sample obtained at 60 °C was characterized by SEM-EDS analysis as well. The result is shown in Figure 10(g) and Table 2. Unlike the morphology obtained at lower temperature, solid pieces with compacted solid phases on the surface were found again. The similar sight with pristine material demonstrated that higher temperature was not suitable for conversion of Sr(OH)2. Although there were some solid particle suspected Sr(OH)2 with high Sr/S ratio, most of particles have almost the same value as the pristine material.
The results obtained in SEM-EDS test confirmed that this conversion reaction was a dissolution-precipitation process. In detail, SrSO4 was accelerated dissolution in high alkaline solution since the generated Sr2+ ions were easily combined with OH– ions at low temperature. A porous Sr(OH)2 membrane which was formed on the surface of celestite ore would not halt the spread of alkaline solution and new products can be continuously formed in the substrate. As a result, the solid of SrSO4 could split and convert to Sr(OH)2 with prolonging time.
4 Conclusions
1) An effective method for Sr/S isolation in SrSO4 conversion process was investigated. The effects of the variables, including initial concentration of NaOH solution, conversion temperature, L/S ratio and leaching time were studied. Results illustrated that the Sr/S ratio of samples increased with initial concentration of NaOH solution, L/S ratio and leaching time, and decreased with conversion temperature.
2) The optimal condition is conversion temperature as 25 °C, initial concentration of NaOH solution as 20 wt%, L/S ratio as 40 mL/g and conversion time as 6 h. The maximum conversion ratio of Sr(OH)2 could reach to 93.88% in optimal condition, and its Sr/S ratio reaches to 41.16. The results showed that most of SrSO4 converted to Sr(OH)2.

Figure 10 SEM images of pre-treated celestite (a, b), sample treated for 1 h (c, d) and sample treated for 6 h (e, f) under condition of initial concentration of NaOH 20 wt%, L/S ratio 40 mL/g, reaction temperature 25 °C and sample treated for 1 h under condition of initial concentration of NaOH of 20 wt%, L/S ratio of 40 mL/g, reaction temperature of 60 °C (g)
3) The reaction mechanism was a dissolution- precipitation process. SrSO4 was dissolved in alkaline solution to divide into Sr2+ and SO42– ions, and Sr(OH)2 would format on the surface of ore particle simultaneously. The porous membrane would not halt the reaction. Cylindrical precipitated which consists of Sr(OH)2 were obtained.
Table 1 EDS parameters of Figure 10(e)

Table 2 EDS parameters of Figure 10(g)
Acknowledgment
The authors also acknowledge Qinghai Zhongkeyuanhao Strontium Technology Co., Ltd. for its partial financial support of this investigation.
References
[1] DUSZA M, STEFANSKI M, WOZNIAK M, HRENIAK D, GERASYMCHUK Y, MARCINIAK L, GRANEK F, STREK W. Luminescent Sr2CeO4 nanocrystals for applications in organic solar cells with conjugated polymers [J]. Journal of Luminescence, 2016, 169: 857–861.
[2] PLAZA M, HUANG X, PETER KO J Y, SHEN M, SIMPSON B H, RODR GUEZ-L
GUEZ-L PEZ J, RITZERT N L, LETCHWORTH-WEAVER K, GUNCELER D, SCHLOM D G, ARIAS T A, BROCK J D, ABRU
PEZ J, RITZERT N L, LETCHWORTH-WEAVER K, GUNCELER D, SCHLOM D G, ARIAS T A, BROCK J D, ABRU A H D. Structure of the photo-catalytically active surface of SrTiO3 [J]. Journal of the American Chemical Society, 2016, 138: 7816–7819.
A H D. Structure of the photo-catalytically active surface of SrTiO3 [J]. Journal of the American Chemical Society, 2016, 138: 7816–7819.
[3] WANG W, JIA D C, ZHOU Y. Preparation and properties of SrBi2.2Ta2O9 thin film [J]. Journal of Central South University, 2005, 12(4): 376–379.
[4] ERDEMO LU M, CANBAZO
LU M, CANBAZO LU M H Y. Carbothermic reduction of high-grade celestite ore to manufacture strontium carbonate [J]. Mineral Processing and Extractive Metallurgy IMM Transactions Section C, 1998, 107: 65–70.
LU M H Y. Carbothermic reduction of high-grade celestite ore to manufacture strontium carbonate [J]. Mineral Processing and Extractive Metallurgy IMM Transactions Section C, 1998, 107: 65–70.
[5] ERDEMO LU M. carbothermic reduction of mechanically activated celestite [J]. International Journal of Mineral Processing, 2009, 92: 144–152.
LU M. carbothermic reduction of mechanically activated celestite [J]. International Journal of Mineral Processing, 2009, 92: 144–152.
[6] YUAN P, HAN H L, DUAN D P. Experimental research on different reductants applied in RHF direct reduction process [J]. Journal of Hebei United University (Natural Science Edition), 2015, 37(1): 52–58. (in Chinese)
[7] SETOUDEH N, WELHAM N J. Ball milling induced reduction of SrSO4 by Al [J]. International Journal of Mineral Processing, 2011, 98(3, 4): 214–218.
[8] SETOUDEH N, WELHAM N J. Mechanochemical reduction of SrSO4 by Mg [J]. International Journal of Mineral Processing, 2012, 104–105: 49–52.
[9] ERDEMO LU M, SARIKAYA M, CANBAZO
LU M, SARIKAYA M, CANBAZO LU M. Leaching of celestite with sodium sulfide [J]. Journal of Dispersion Science and Technology, 2006, 27: 439–442.
LU M. Leaching of celestite with sodium sulfide [J]. Journal of Dispersion Science and Technology, 2006, 27: 439–442.
[10] ZORAGA M, KAHRUMAN C, YUSUFOGLU I. Conversion kinetics of SrSO4 to SrCO3 in solution obtained by dissolving/hydrolyzing of equimolar amounts of NH4HCO3 and NH4COONH2 [J]. Hydrometallurgy, 2016, 163: 120–129.
[11] BINGOL D, AYDOGAN S, GULTEKIN S S. Neural model for the leaching of celestite in sodium carbonate solution [J]. Chemical Engineering Journal, 2010, 165: 617–624.
[12] SETOUDEH N, WELHAN N J, AZAMI S M. Dry mechanochemical conversion of SrSO4 to SrCO3 [J]. Journal of Alloys and Compounds, 2010, 492: 389–391.
[13] AYDO AN S, ERDEMO
AN S, ERDEMO LU M, ARAS A, U
LU M, ARAS A, U AR G,
AR G,  ZKAN A. Dissolution kinetics of celestite (SrSO4) in HCl solution with BaCl2 [J]. Hydrometallurgy, 2006, 84: 239–246.
ZKAN A. Dissolution kinetics of celestite (SrSO4) in HCl solution with BaCl2 [J]. Hydrometallurgy, 2006, 84: 239–246.
[14] SU REZ-ORDUNA R, REND
REZ-ORDUNA R, REND N-ANGELES J C, MATAMOROS-VELOZA Z, YANAGISAWA K. Exchange of SO42- ions with F– ions in mineral celestite under hydrothermal conditions [J]. Solid State Ionics, 2004, 172(1–4): 393–396.
N-ANGELES J C, MATAMOROS-VELOZA Z, YANAGISAWA K. Exchange of SO42- ions with F– ions in mineral celestite under hydrothermal conditions [J]. Solid State Ionics, 2004, 172(1–4): 393–396.
[15] REND N-ANGELES J C, MATAMOROS-VELOZA Z, VELOZA A M, PEREZ-GARIBAY R, RODRIGUEZ- GALICIA J, KAZUMICHI Y. Facile synthesis of perovskite- structured powders using barite–celestite ore under hydrothermal alkaline conditions [J]. Industrial & Engineering Chemistry Research, 2017, 56(36): 9942–9952.
N-ANGELES J C, MATAMOROS-VELOZA Z, VELOZA A M, PEREZ-GARIBAY R, RODRIGUEZ- GALICIA J, KAZUMICHI Y. Facile synthesis of perovskite- structured powders using barite–celestite ore under hydrothermal alkaline conditions [J]. Industrial & Engineering Chemistry Research, 2017, 56(36): 9942–9952.
[16] ZORAGA M, KAHRUMAN C, YUSUFOGLU I. Determination of conversion reaction mechanism of celestite to acidic strontium oxalate hydrate in aqueous solution of H2C2O4 [J]. Hydrometallurgy, 2017, 171: 53–60.
[17] TURIANICOV E, OBUT A, ZORKOVSK
E, OBUT A, ZORKOVSK A, BAL
A, BAL
 P, MATIK M, BRIAN
P, MATIK M, BRIAN IN J. The effects of LiOH and NaOH on the carbonation of SrSO4 by dry high-energy milling [J]. Minerals Engineering, 2013, 49: 98–102.
IN J. The effects of LiOH and NaOH on the carbonation of SrSO4 by dry high-energy milling [J]. Minerals Engineering, 2013, 49: 98–102.
[18]  AHIN M, ERDEM M. Cleaning of high lead-bearing zinc leaching residue by recovery of lead with alkaline leaching [J]. Hydrometallurgy, 2015, 153: 170–178.
AHIN M, ERDEM M. Cleaning of high lead-bearing zinc leaching residue by recovery of lead with alkaline leaching [J]. Hydrometallurgy, 2015, 153: 170–178.
[19] LIU Y, ZHANG Y F, CHEN F F, ZHANG Y. The alkaline leaching of molybdenite flotation tailings associated with galena [J]. Hydrometallurgy, 2012, 129–130: 30–34.
[20] SONG L M, LI Y M, HE P Z, ZHANG S J, WU X Q, FANG S, SHAN J J, SUN D L. Synthesis and sonocatalytic property of rod-shape Sr(OH)2·8H2O [J]. Ultrasonics Sonochemistry, 2014, 21: 1318–1324.
[21] KADARI A, MAHI K, MOSTEFA R, BADAOUI M, MAMECHE A, KADRI D. Optical and structural properties of Mn doped CaSO4 powders synthesized by sol-gel process [J]. Journal of Alloys and Compounds, 2016, 688: 32–36.
[22] LOPEZ-VALDIVIESO A, ROBLEDO-CABRERA A, URIBE-SALAS A. Flotation of celestite with the anionic collector sodium dodecyl sulfate [J]. Int J Miner Process, 2000, 60: 79–90.
[23] OWUSU G, LITZ J E. Water leaching of SrS and precipitation of SrCO3 using carbon dioxide as the precipitating agent [J]. Hydrometallurgy, 2000, 57: 23–29.
(Edited by HE Yun-bin)
中文导读
天青石(SrSO4)碱性溶液转化Sr(OH)2过程中的Sr/S元素高效分离
摘要:天青石制备其他锶化合物的瓶颈在于如何实现Sr/S元素的高效分离。本文针对天青石转化Sr(OH)2过程中的Sr/S元素高效分离进行研究,开发出一种低能耗及零污染的Sr/S元素分离新方法,其中Sr元素以Sr(OH)2形式存在于固相中,S元素则以Na2SO4形式进入液相中。通过对反应过程中的因素,如初始NaOH浓度、转化温度、液固比和转化时间对Sr/S元素分离效率的影响进行的研究,结果发现Sr/S元素的分离效率随着初始NaOH浓度、液固比和反应时间的增大而增加,而随着反应温度的升高而降低。在所得最优转化条件下,Sr(OH)2的最大转化率可达93.88%,该样品中的Sr/S比可达到41.16。这证实了在碱性溶液中天青石转化成Sr(OH)2过程可以较好地实现Sr/S的高效分离。样品的SEM-EDS测试结果表明其转化过程为溶解-沉积过程。
关键词:天青石;Sr(OH)2;Sr/S元素高效分离;溶解-沉积过程
Foundation item: Project(2015-GX-108A) supported by Qinghai Provincial Science and Technology Support Program, China
Received date: 2017-11-12; Accepted date: 2018-10-11
Corresponding author: DUAN Dong-ping, PhD, Professor; Tel: +86-10-82544916; E-mail: dongping_duan@263.net; ORCID: 0000- 0003-3821-2124

