基于石灰苛化法研究CaO在含草酸盐稀铝酸钠溶液中的反应动力学与机理
来源期刊:中国有色金属学报(英文版)2019年第6期
论文作者:张佰永 潘晓林 王江洲 于海燕 涂赣峰
文章页码:1312 - 1322
关键词:拜耳法;石灰苛化;草酸盐;动力学;铝酸钠溶液
Key words:Bayer process; lime causticization; oxalate; kinetics; sodium aluminate solution
摘 要:基于草酸盐石灰苛化法研究水合铝酸三钙和草酸钙分别在稀铝酸钠溶液和草酸钠溶液中的反应动力学和机理,并获得铝酸钠溶液去除草酸盐的最佳条件及其去除机理。水合铝酸三钙和草酸钙的生成分别受化学反应控制和内扩散控制,分别计算其反应速率方程和表观活化能。在含草酸盐的稀铝酸钠溶液中能够形成具有空间交错片状结构的水铝钙石相,其通过吸附大幅度降低溶液中草酸盐杂质。随着反应时间的延长,生成的草酸钙能够转化为水合铝酸三钙。在反应温度为50 °C、搅拌速度为200 r/min下,草酸盐苛化效率达到90%以上,氧化铝损失率在31%以下。
Abstract: The formation kinetics and mechanism of tricalcium aluminate hydrate and calcium oxalate in dilute sodium aluminate solution and sodium oxalate solution were studied respectively based on the lime causticization, and the optimal conditions for removing the oxalate in dilute sodium aluminate solution as well as the mechanism were finally obtained. The formation processes of tricalcium aluminate hydrate and calcium oxalate are mainly controlled by the chemical reaction and the inner diffusion respectively, and the corresponding reaction rate equations as well as the apparent activation energy were calculated. The hydrocalumite with a spatially interleaved structure will form in dilute sodium aluminate solution with sodium oxalate, greatly removing the oxalate impurity by absorption. Calcium oxalate can be converted to tricalcium aluminate hydrate with the increasing reaction time. The oxalate causticization efficiency and the alumina loss rate can be over 90% and below 31% respectively when reacted at 50 °C with a stirring rate of 200 r/min.
Trans. Nonferrous Met. Soc. China 29(2019) 1312-1322
Bai-yong ZHANG1,2, Xiao-lin PAN1, Jiang-zhou WANG1, Hai-yan YU1, Gan-feng TU1
1. School of Metallurgy, Northeastern University, Shenyang 110819, China;
2. Shenyang Aluminum & Magnesium Engineering & Research Institute Co. Ltd., Shenyang 110001, China
Received 20 September 2018; accepted 3 April 2019
Abstract: The formation kinetics and mechanism of tricalcium aluminate hydrate and calcium oxalate in dilute sodium aluminate solution and sodium oxalate solution were studied respectively based on the lime causticization, and the optimal conditions for removing the oxalate in dilute sodium aluminate solution as well as the mechanism were finally obtained. The formation processes of tricalcium aluminate hydrate and calcium oxalate are mainly controlled by the chemical reaction and the inner diffusion respectively, and the corresponding reaction rate equations as well as the apparent activation energy were calculated. The hydrocalumite with a spatially interleaved structure will form in dilute sodium aluminate solution with sodium oxalate, greatly removing the oxalate impurity by absorption. Calcium oxalate can be converted to tricalcium aluminate hydrate with the increasing reaction time. The oxalate causticization efficiency and the alumina loss rate can be over 90% and below 31% respectively when reacted at 50 °C with a stirring rate of 200 r/min.
Key words: Bayer process; lime causticization; oxalate; kinetics; sodium aluminate solution
1 Introduction
Sodium oxalate in industrial sodium aluminate solution is mainly decomposed from the organics in the Bayer process, which originally comes from the organic compounds (humus) in bauxite and the organic additives during the alumina production, including flocculants, defoamers and dehydrating agents [1,2]. When the oxalate accumulates and exceeds a critical concentration, it will greatly affect the normal operation of the Bayer process [3]. As the oxalate is easy to crystallize, it can act as the secondary crystal core of aluminum hydroxide during the seed precipitation, which leads to the particle refinement and the chemical alkali increase in the decomposition products [4,5]. Meanwhile, the oxalate will also form brown layered scaling on the inner wall of decomposition equipment and evaporators, resulting in the blockage of pipes and the decrease of heat transfer efficiency [6]. In addition, the presence of oxalate increases the viscosity, decreases the surface tension of sodium aluminate solution, and decreases the settling property of red mud [7,8].
At present, the main methods of removing sodium oxalate include crystallization [9], oxidation [10-12], ion exchange [13], adsorption [14] and lime causticization [15-17]. Although each method has advantages and disadvantages, the lime causticization is recognized as the most prominent method because of its low operation cost, simple operation and high caustic soda recovery. However, due to the existence of caustic alkali and alumina in washing solution, tricalcium aluminate hydrate (TCA) and calcium oxalate will generate synchronously, which leads to a large amount of lime addition and low lime utilization rate. ROSENBERG et al [15] proposed a two-stage lime causticization process, i.e. the first stage to remove the aluminate ions by the formation of TCA, and the second stage to remove the oxalate with additional calcium oxide. They even increased the addition of calcium oxide from 20% to 80% in the subsequent study followed by the recovery of alumina by adding carbonate [16]. LIU et al [18] investigated the behavior of calcium oxalate in sodium aluminate solution containing sodium carbonate, and the results showed that calcium oxalate can be converted to TCA and calcium carbonate in sodium aluminate solution and sodium carbonate solution, respectively. ROSENBERG and ARMSTRONG [19] and PEROTTA et al [20,21] reported that the layered double hydroxides, such as hydrocalumite (Ca4Al2CO9·11H2O) and hydrotalcite (Mg6Al2(OH)16CO3.4H2O), can offer a potentially new and efficient option for organic and inorganic impurity removal from alumina refinery liquors by adsorption, especially for carbonate or oxalate.
Most of the above studies focused on the improvement of the causticizing conditions, but the kinetics and mechanism of calcium oxalate formation were paid less attention in dilute sodium aluminate solution during the causticizing process. In this work, the formation kinetics and mechanism of TCA and calcium oxalate in dilute sodium aluminate solution and sodium oxalate solution were firstly studied, and then the formation mechanism of calcium oxalate in sodium aluminate solution with sodium oxalate was discussed.
2 Experimental
In order to eliminate the influence of impurity ions in industrial sodium aluminate solution, analytical reagents were used in this work. The reaction behaviors of calcium oxide in three liquor systems were studied, i.e. sodium aluminate solution (CaO-NaAl(OH)4) system), sodium oxalate solution (CaO-Na2C2O4 system), and sodium aluminate solution with sodium oxalate (CaO-NaAl(OH)4-Na2C2O4 system). Sodium aluminate solution was prepared by adding Al(OH)3 into the boiled NaOH solution, and CaO was prepared by calcining CaCO3 at 1050 °C for 3 h. The concentration of sodium aluminate solution is as follows: caustic soda (CNK, in form of Na2O) is 25.0 g/L, alumina (CAO) is 14.8 g/L, sodium oxalate (CSO) is 4.0 g/L.
The reaction experiments of calcium oxide with different liquor systems were carried out in three necked flasks under different conditions. The slurry after reaction was separated by filtration. The filter cake was washed carefully and dried for tests, while the concentration of filtrate was determined by the volumetric method. The alumina concentration was titrated by EDTA and lead acetate solution using xylenol orange as an indicator [22], and the oxalate concentration was titrated by ceric sulfate using phenanthroline as an indicator [23].
The conversion rate of TCA (ηTCA) in CaO- NaAl(OH)4 system was calculated by
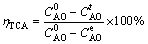 (1)
(1)
where  ,
,  and
and 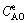 are the alumina concentrations at the initial stage, reaction time t and reaction equilibrium, respectively.
are the alumina concentrations at the initial stage, reaction time t and reaction equilibrium, respectively.
The conversion rate of calcium oxalate (ηCO) in CaO-Na2C2O4 system was calculated by
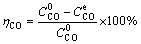 (2)
(2)
where  and
and 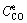 are the sodium oxalate concentrations at the initial stage and reaction equilibrium, respectively.
are the sodium oxalate concentrations at the initial stage and reaction equilibrium, respectively.
The causticization efficiency (ηCO) and alumina loss rate (ηAO) in CaO-NaAl(OH)4-Na2C2O4 system were calculated by Formulas (2) and (3), respectively:
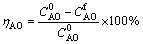 (3)
(3)
where  and
and 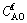 are the initial concentration and the final concentration of Al2O3 in sodium aluminate solution.
are the initial concentration and the final concentration of Al2O3 in sodium aluminate solution.
X-ray diffraction measurements with an X-ray powder diffractometer (XRD, PANalytical PW3040/60) using Cu Kα radiation were performed to identify the minerals in solid samples with a scan rate of 3 (°)/min. Scanning electron microscopy and energy dispersive X-ray spectroscopy (EDX) were performed using a scanning electron microscope (FESEM, Zeiss UltraPlus), operating at an accelerating voltage of 15 kV. Solid powders were deposited on a Cu support plate with Au support film (conductive coating, deposited by low vacuum sputter coating). A laser particle size analyzer (Malvern 3000) was applied to determining the particle size distribution.
3 Results and discussion
3.1 Reaction behavior of calcium oxide in dilute sodium aluminate solution
The reaction process of calcium oxide in dilute sodium aluminate solution at different temperatures for different durations under the lime causticization conditions was firstly studied. The concentration of calcium oxide added to sodium aluminate solution is 10.0 g/L in order to guarantee the excess of calcium oxide during the reaction, and the stirring rate is 150 r/min. The alumina concentrations of sodium aluminate solution reacted with calcium oxide under different conditions are listed in Table 1, and the corresponding conversion rates of TCA were calculated as shown in Fig. 1.
As shown in Fig. 1, the conversion rate of TCA in dilute sodium aluminate solution depends on the reaction temperature, which greatly increases with the rising temperature, and the corresponding time to reach the reaction equilibrium is also greatly reduced. At 70 °C, the reaction equilibrium can be finished only in 5 min.
Table 1 Concentrations of alumina in sodium aluminate solution after TCA formation under different conditions

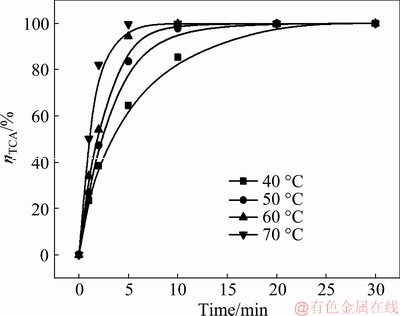
Fig. 1 Conversion rate of TCA under different reaction conditions
The reaction between calcium oxide and sodium aluminate solution is a simple liquid-solid reaction, which corresponds to the unreacted shrinkage nucleus model. The reaction process may be controlled by the chemical reaction or internal diffusion, and the corresponding reaction kinetic equations are shown as follows:
 (4)
(4)
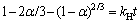 (5)
(5)
where kH is the reaction rate constant, a is the conversion rate of TCA (ηTCA).
The values of 1-(1-α)1/3 and 1-2α/3-(1-α)2/3 were calculated based on the conversion rate of TCA at different temperatures. The relationship between 1-(1-α)1/3 or 1-2α/3-(1-α)2/3 and reaction time is shown in Fig. 2. It shows that the first kinetic models fit well, and the fitting correlation coefficient is more than 0.97, which indicates that the formation of TCA is controlled by the chemical reaction.
The reaction rate constants of kH are listed in Table 2, which are the slopes of the straight line after fitting under different temperatures according to Fig. 2 (a).
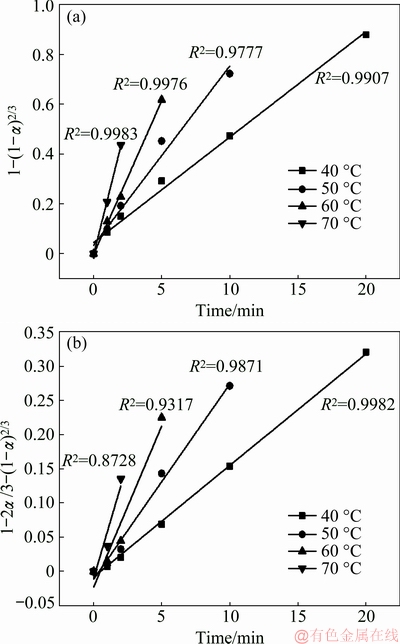
Fig. 2 Relationship between 1-(1-α)1/3 (a) or 1-2α/3-(1-α)2/3 (b) and reaction time for TCA formation
Table 2 Reaction rate constants of TCA formation in dilute sodium aluminate solution

According to the Arrhenius equation as shown in Eq. (6) (EH is the apparent activation energy, kJ/mol, A is the pre-exponential factor), the relationship between ln kH (see in Table 2) and 1/T for the TCA formation is shown in Fig. 3. The activation energy was calculated to be 48.54 kJ/mol, and the pre-exponential factor is 5.23×106. Accordingly, the rate equation for the formation of TCA in dilute sodium aluminate solution is shown in Eq. (7).
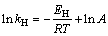 (6)
(6)
1-(1-α)1/3=5.23×106×exp[48540/(RT)] (7)
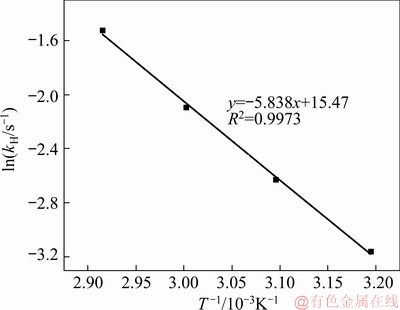
Fig. 3 Relationship between ln kH and 1/T for TCA formation
It was reported that when the activation energy is between 42 and 800 kJ/mol, the reaction is controlled by the chemical reaction [24]. Therefore, the reaction of calcium oxide in dilute sodium aluminate solution is mainly subject to the chemical reaction.
The XRD patterns of the reacted products of calcium oxide with sodium aluminate solution at different temperatures for 30 min are shown in Fig. 4. The products are mainly comprised of TCA and a small amount of hydrocalumite. The formation of hydro- calumite is due to the existence of CO2 in air which enters into the solution during the reaction, as listed in Eq. (8).
Ca(OH)2+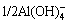 +
+ +5/4H2O=1/4(Ca4Al2CO9·11H2O)+OH- (8)
+5/4H2O=1/4(Ca4Al2CO9·11H2O)+OH- (8)
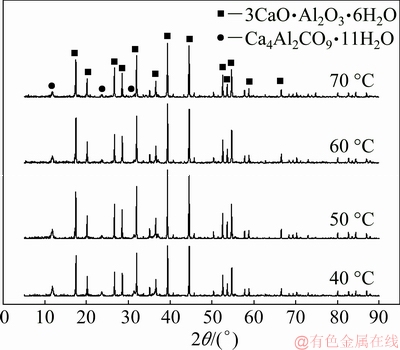
Fig. 4 XRD patterns of reacted products of calcium oxide in sodium aluminate solution at different temperatures for 30 min
The microstructure of the reacted product of calcium oxide with sodium aluminate solution at 50 °C for 30 min and the corresponding EDX spectra are shown in Fig. 5. The particle size of the product ranges from several to tens of microns as seen in Fig. 5(a), precipitating in form of agglomerates (Fig. 5(b)). As seen from the higher-magnification images, most of the reacted products form in the morphology of spheroidal particles (Fig. 5(c)), and some disc particles also exist (Fig. 5(d)). The EDX spectra of the above particles are shown in Figs. 5(e) and (f), respectively. The composition of carbon in the disc particle is much higher than that of spheroidal particle, indicating that the spheroidal particles are TCA and the disc particles are hydrocalumite.
3.2 Reaction behavior of calcium oxide in sodium oxalate solution
The reaction process of calcium oxide in sodium oxalate solution under the lime causticization conditions was then studied. The experiments were carried out at the same temperatures in sodium aluminate solution for different durations. The initial concentration of sodium oxalate is 4.0 g/L, and the stirring rate is 150 r/min. Calcium oxide was added to sodium oxalate solution with the same molar ratio.
The concentrations of oxalate reacted with calcium oxide under different conditions are listed in Table 3, and the corresponding conversion rates of calcium oxalate were calculated as shown in Fig. 6.
Figure 6 shows that sodium oxalate reacts with calcium oxide very fast in the first 15 min at different temperatures, and then it reacts slowly due to the low solubility of calcium oxide in solution. When calcium oxide is added, the contact area of reactants is large and the reaction goes fast. As the dissolution rate of calcium oxide becomes slow, the remaining reaction becomes slow. Meanwhile, as the reaction temperature increases, the conversion rate of calcium oxalate increases.
The values of 1-(1-α)1/3 and 1-2α/3-(1-α)2/3 were calculated based on the conversion rate of calcium oxalate at different temperatures as shown in Fig. 7.
The reaction rate curves in Fig. 7(b) basically pass through the origin, and the linear fitting correlation coefficients (R2) are much higher than those in Fig. 7(a), indicating that the reaction of sodium oxalate and calcium oxide may be subject to the inner diffusion. Based on Fig. 7(b), the apparent rate constants (kH) at various temperatures were calculated as shown in Table 4, which increase with the increasing reaction temperature.
According to Fig. 8, the activation energy was calculated to be 72.75 kJ/mol, and the pre-exponential factor is 1.39×108. The rate equation for the reaction of calcium oxide in sodium oxalate solution is as follows:
1-2α/3-(1-α)2/3=1.39×108×exp[72750/(RT)] (9)
According to the results of kinetics fitting, the reaction of calcium oxide in sodium oxalate solution is mainly controlled by the inner diffusion, which is not consistent with its activation energy. The reason may be due to the small solubility of calcium oxide in aqueous solution and the surface coating by insoluble calcium compounds, which makes the reaction rate slower and the activation energy higher.

Fig. 5 SEM images of reacted product of calcium oxide in sodium aluminate solution at 50 °C for 30 min with different magnifications (a, b, c, d) and EDX spectra (e, f) corresponding to (c, d), respectively
Table 3 Concentrations of oxalate after reaction with calcium oxide under different conditions
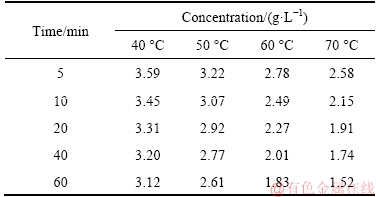

Fig. 6 Conversion rates of calcium oxalate under different reaction conditions

Fig. 7 Relationship between 1-(1-α)1/3 (a) or 1-2α/3-(1-α)2/3 (b) and reaction time for calcium oxalate formation
Table 4 Reaction rate constants of calcium oxalate formation


Fig. 8 Relationship between ln kH and 1/T for calcium oxalate formation
The XRD patterns of the reacted products of calcium oxide in sodium oxalate solution at different temperatures for 60 min are shown in Fig. 9. Calcium oxalate (CaC2O4·H2O) is the main product, and some unreacted calcium hydroxide (CaO·H2O) still exists. The diffraction peak intensity of calcium oxalate increases with the increasing reaction temperature, indicating that high temperature promotes the formation rate of calcium oxalate, which is consistent with the results shown in Fig. 7.

Fig. 9 XRD patterns of reacted products of calcium oxide in sodium oxalate solution at different temperatures for 60 min
The microstructure of the reacted product of calcium oxide in sodium oxalate solution at 50 °C for 60 min and the corresponding EDX spectrum are shown in Fig. 10. The formed particles are bigger than those precipitated in sodium aluminate solution as shown in Fig. 10(a). Most of the particles are rod-like in form of agglomerates (Fig. 10(b)), referring to calcium oxalate, which is also confirmed by the EDX result as shown in Fig. 10(d). The morphology of calcium hydroxide as shown in Fig. 10(c) is different from that of TCA, and the particle size is much smaller than that of TCA.
3.3 Reaction behavior of calcium oxide in sodium aluminate solution with sodium oxalate

Fig. 10 SEM images (a, b, c) of reacted product of calcium oxide in sodium oxalate solution at 50 °C for 60 min and corresponding EDX spectrum (d) of calcium oxalate
In fact, in order to eliminate the oxalate impurity, the reactions of Sections 3.1 and 3.2 coexist in sodium aluminate solution with sodium oxalate during the lime causticization process, as listed in Eqs. (8), (10) and (11). LIU et al [18] calculated the formation thermodynamics of calcium oxalate, hydrocalumite, calcium carbonate and TCA, and also studied the stability of calcium oxalate in sodium carbonate solution or sodium aluminate solution. They pointed out that calcium oxalate will transform to hydrocalumite, calcium carbonate and TCA when the reaction temperature, residence time and caustic soda concentration increase. WHITTINGTON and CARDILE [25] and SALIMI et al [26] studied the phase diagrams and solubility of TCA in synthetic Bayer liquor respectively, and TCA was found to form more easily at low temperatures, caustic concentrations and carbonate concentrations.
Ca(OH) 2+2/3NaAl(OH)4(aq)=1/3(3CaO·Al2O3·6H2O)+2/3NaOH(aq) (10)
Ca(OH)2+Na2C2O4(aq)=CaC2O4·H2O+2NaOH(aq) (11)
The formation of calcium oxalate in Eq. (11) can remove the oxalate impurity in sodium aluminate solution, but the formation of TCA in Eqs. (8) and (10) inevitably causes the loss of alumina. According to the dynamics studies, the formation of TCA is mainly controlled by the chemical reaction, while the formation of calcium oxalate is mainly controlled by the inner diffusion. Therefore, the effects of temperature and stirring rate on the causticization efficiency and alumina loss rate in sodium aluminate solution with sodium oxalate were studied.
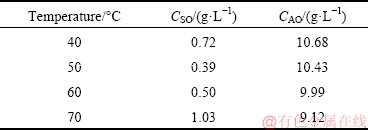
The reaction process of calcium oxide in sodium aluminate solution with sodium oxalate at different temperatures was firstly studied. The concentration of sodium oxalate in sodium aluminate solution is 4.0 g/L, the concentration of calcium oxide added to the solution is 10.0 g/L, the reaction time is 60 min, and the stirring rate is 150 r/min. The concentrations of sodium oxalate and alumina after reaction are listed in Table 5. As the reaction temperature increases from 40 to 70 °C, the concentration of sodium oxalate decreases firstly and then increases gradually, while the concentration of alumina decreases. The causticization efficiency and alumina loss rate were calculated according to Table 5 as shown in Fig. 11. The causticization efficiency first increases and then decreases with the increasing temperature, and the maximum value is 90.33% at 50 °C. The alumina loss rate increases gradually with the increasing temperature.
Table 5 Concentrations of sodium oxalate and alumina after reaction of calcium oxide in sodium aluminate solution containing sodium oxalate at different temperatures for 60 min
The reaction process of calcium oxide in sodium aluminate solution with sodium oxalate at different stirring rates was then studied. The concentration of sodium oxalate in sodium aluminate solution is 4.0 g/L, the concentration of calcium oxide added to the solution is 10.0 g/L, the reaction temperature is 50 °C, and the reaction time is 60 min. The concentrations of sodium oxalate and alumina after reaction are listed in Table 6, and the corresponding causticization efficiency and alumina loss rate are shown in Fig. 12. The effect of stirring rate on the causticization efficiency is great. The causticization efficiency first increases and then decreases with the increasing stirring rate, and reaches the maximum of 90.33% when the stirring rate is 200 r/min. As the stirring rate is less than 200 r/min, the causticization efficiency increases slightly with the increasing stirring rate; as the stirring rate continues to increase, it will lead to the generation of large amount of TCA at the early stage, resulting in a great decrease of causticization efficiency at higher stirring rates.

Fig. 11 Causticization efficiency and alumina loss rate of calcium oxide in sodium aluminate solution containing sodium oxalate at different temperatures for 60 min
Table 6 Concentrations of sodium oxalate and alumina after reaction of calcium oxide in sodium aluminate solution containing sodium oxalate at different stirring rates

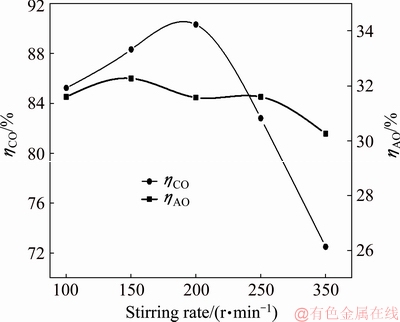
Fig. 12 Causticization efficiency and alumina loss rate of calcium oxide in sodium aluminate solution containing sodium oxalate at different stirring rates
According to the above results, the reaction temperature and stirring rate should be strictly controlled when removing sodium oxalate from sodium aluminate solution. The causticization efficiency is over 90% and the alumina loss rate is below 31% when the reaction temperature is 50 °C with a stirring rate of 200 r/min.
The reaction mechanism of calcium oxide in sodium aluminate solution with sodium oxalate for different reaction time was finally studied. The concentration of sodium oxalate in sodium aluminate solution is 4.0 g/L, the concentration of calcium oxide added to the solution is 10.0 g/L, the reaction temperature is 50 °C, and the stirring rate is 200 r/min. The concentrations of sodium oxalate and alumina after reaction decrease with the increasing reaction time as listed in Table 7. As shown in the XRD patterns of the reacted products in Fig. 13, although calcium hydroxide is the main phase, hydrocalumite and calcium oxalate also form at the initial formation stage according to Eqs. (8) and (11) respectively. As the reaction proceeds, the amount of hydrocalumite increases, while the amount of calcium hydroxide decreases. When the reaction time increases to 60 min, calcium oxalate disappears, and some TCA forms.
Table 7 Concentrations of sodium oxalate and alumina after reaction of calcium oxide in sodium aluminate solution containing sodium oxalate for different reaction time

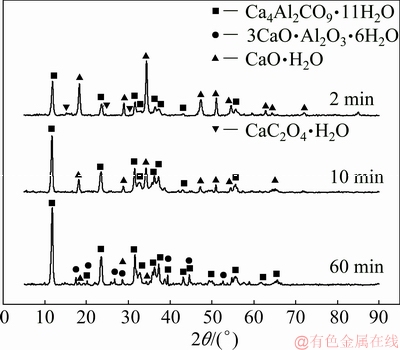
Fig. 13 XRD patterns of reacted products of calcium oxide in sodium aluminate solution containing sodium oxalate at 50 °C for different time
According to the results of LIU et al [18], the carbonate in sodium aluminate solution favors the formation of hydrocalumite and TCA instead of calcium carbonate, and significantly increases the conversion ratio of calcium oxalate; meanwhile, calcium oxalate can be converted to TCA in sodium aluminate solution at elevated temperature as listed in Eq. (12). The experimental results in this study also confirm the above conclusions.
3CaC2O4·H2O+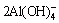 +4OH-=3CaO·Al2O3·6H2O+
+4OH-=3CaO·Al2O3·6H2O+ +3H2O (12)
+3H2O (12)
The diffraction intensities of hydrocalumite increase with increasing the reaction time, indicating that the amount of hydrocalumite increases accordingly. When the time is 60 min the total carbon content of the reacted product was determined to be 3.45%, among which the carbon content of oxalate is 2.65%, indicating that the hydrocalumite can absorb a large number of oxalate in solution because of its layered double structure [19-21]. Thus, the concentration of sodium oxalate decreases as the reaction proceeds as listed in Table 7.
The particle size distribution and parameters of products formed at 50 °C for different time are shown in Fig. 14 and Table 8. The particle size increases with the increasing reaction time, and a bimodal distribution appears. The reason is maybe that the solid grains grow gradually and the grain aggregation becomes more and more obvious with the increasing reaction time.
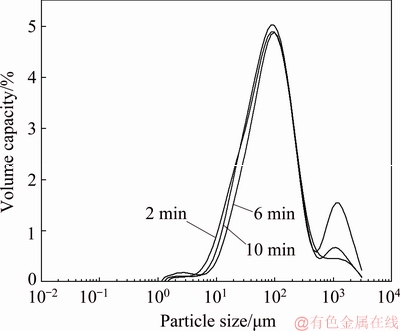
Fig. 14 Particle size distribution of reacted products of calcium oxide in sodium aluminate solution containing sodium oxalate at 50 °C for different time
Table 8 Particle size parameters of reacted products of calcium oxide in sodium aluminate solution containing sodium oxalate at 50 °C for different time

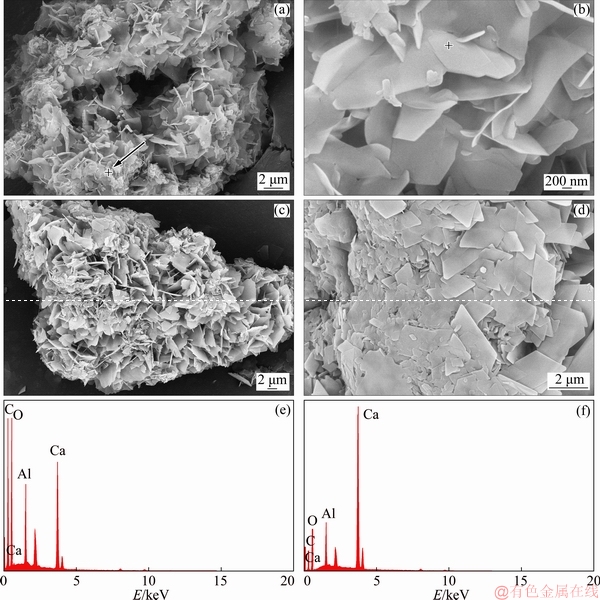
Fig. 15 SEM images of reacted products of calcium oxide in sodium oxalate solution containing sodium oxalate at 50 °C for 2 min (a, b), 60 min (c, d) and corresponding EDX spectra (e, f)
The microstructures of the products reacted for different reaction time are shown in Fig. 15. At the initial formation stage, the hydrocalumite with a sheet structure forms extensively and quickly as shown in Fig. 15(b) (its EDX spectrum is shown in Fig. 15(e)), resulting in lots of holes during the agglomeration process (Fig. 15(a)). Meanwhile, the sheet structure is irregular, which is similar to the morphology of hydrocalumite in dilute sodium aluminate solution as shown in Fig. 5(d). The unreacted calcium hydroxide with a spheroidal structure still exists as shown by the arrow in Fig. 15(a), and its EDX spectrum is shown in Fig. 15(f). At the end of reaction as shown in Figs. 15(c) and (d), the sheet structure of hydrocalumite forms more compactly and completely, which presents a diamond morphology.
4 Conclusions
(1) The reaction of calcium oxide in dilute sodium aluminate solution is mainly controlled by the chemical reaction, and the reaction rate equation of TCA is 1-(1-α)1/3=5.23×106×exp[48540/(RT)]; the reaction of calcium oxide in sodium oxalate solution is mainly controlled by the inner diffusion, and the reaction rate equation of calcium oxalate is 1-2α/3-(1-α)2/3= 1.39×108×exp[72750/(RT)].
(2) The hydrocalumite with a spatially interleaved structure will form in dilute sodium aluminate solution with sodium oxalate, greatly removing the oxalate impurity by absorption. Calcium oxalate can be converted to TCA with the increasing reaction time.
(3) The optimal removal conditions of oxalate in dilute sodium aluminate solution containing oxalate were proposed to react at 50 °C with a stirring rate of 200 r/min, the causticization efficiency and the alumina loss rate of which can be over 90.33% and below 31%, respectively.
References
[1] KIM M J, LEE S O. Overview of the behaviour of sodium oxalate in Bayer liquor and its effect of the process [C]//Light Metals. San Diego, California: TMS, 2003: 19-24.
[2] MACHOLD T, LAIRD D W, ROWEN C C. Decomposition of Bayer process organics: Phenolates, polyalcohols, and additional carboxylates [J]. Hydrometallurgy, 2011, 107(3-4): 68-73.
[3] WELLINGTON M, VALCIN F. Impact of Bayer process liquor impurities on causticization [J]. Industrial & Engineering Chemistry Research, 2007, 46(15): 5094-5099.
[4] SMEULDERS D E, WILSON M A, ARMSTRONG L. Insoluble organic compounds in the Bayer process [J]. Industrial & Engineering Chemistry Research, 2001, 40(10): 2243-2251.
[5] BUSETTI F, BERWICK L, MCDONALD S. Physicochemical characterization of organic matters in Bayer liquor [J]. Industrial & Engineering Chemistry Research, 2014, 53(15): 6544-6553.
[6] POWER G, LOH J S, VERNON C. Organic compounds in the processing of lateritic bauxites to alumina. Part 2: Effects of organics in the Bayer process [J]. Hydrometallurgy, 2012, 127-128: 125-149.
[7] POWER G, LOH J. Organic compounds in the processing of lateritic bauxites to alumina. Part 1: Origins and chemistry of organics in the Bayer process [J]. Hydrometallurgy, 2010, 105(1-2): 1-29.
[8] WANG Meng, HU Hui-ping, LIU Jin-wen. Negative effects of dissolved organic compounds on settling performance of goethite in Bayer red mud [J]. Transaction of Nonferrous Metals Society of China, 2017, 27(2): 429-439.
[9] THE P J, BUSH J F. Solubility of sodium oxalate in Bayer liquor and a method of control [C]//Light Metals. Denver, CO: TMS, 1987: 5-10.
[10] ARNSWALD W, KALTENBERG H G, GUHL E. Removal of organic carbon from Bayer liquor by wet oxidation in tube digesters [C]//Light Metals. New Orleans, LA: TMS, 1991: 23-27.
[11] TARDIO J, BHARGAVA S, EYER S. Interactions between specific organic compounds during catalytic wet oxidation of Bayer liquor [J]. Industrial & Engineering Chemistry Research, 2004, 43(4): 847-851.
[12] LOH J S C, BRODIE G M, POWER G. Wet oxidation of precipitation yield inhibitors in sodium aluminate solutions: Effects and proposed degradation mechanisms [J]. Hydrometallurgy, 2010, 104(2): 278-289.
[13] HIND A R, BHARGAVA S K, GROCOTT S C. The surface chemistry of Bayer process solids: A review [J]. Colloids Surface A: Physicochemical & Engineering Aspects, 1999, 146(1): 359-374.
[14] PERROTTA A J, WILLIAMS F. Hydrocalumite formation in Bayer liquor and its promotional effect on oxalate precipitation [C]//Light Metals. Warrendale, Pennsylvania: TMS, 1995: 77-87.
[15] ROSENBERG S P, TICHBON W, WILSON D J, HEATH C A. Process for the removal of oxalate and /or sulphate from Bayer liquors: US6743403[P]. 2004-06-01.
[16] ROSENBERG S P, WILSON D J, HEATH C A. Inhibiting the formation of TCA in a Bayer causticisation process: US7767190[P]. 2010-08-03.
[17] ROSENBERG S P, WILSON D J, HEATH C A. Some aspects of calcium chemistry in the Bayer process [C]//Light Metals. New Orleans, LA: TMS, 2001: 19-24.
[18] LIU Gui-hua, DONG Wen-bo, QI Tian-gui. Behavior of calcium oxalate in sodium aluminate solutions [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(8): 1878-1887.
[19] ROSENBERG S P, ARMSTRONG L. Layered double hydroxides in the Bayer process: past, present and future [C]//Light Metals. San Francisco, CA: TMS, 2005: 157-161.
[20] PERROTTA A J, WILLIAMS F. Layered double hydroxide formation in Bayer liquor and its promotional effect on oxalate precipitation [C]//Light Metals. Anaheim, CA: TMS, 1996: 17-28.
[21] PEROTTA A J, WILLIAMS F S, STONEHOUSE L. Layered double hydroxides for treatment of Bayer process lake water [C]//Light Metals. Orlando, FL: TMS, 1997: 37-48.
[22] PAN Xiao-lin, YU Hai-yan, TU Gan-feng, BI Shi-wen. Effects of precipitation activity of desilication products (DSPs) on stability of sodium aluminate solution [J]. Hydrometallurgy, 2016, 165: 261-269.
[23] WU Xiao-ling. The method of measuring sodium oxalate by ceric sulfate [J]. Journal of Xinjiang Medical University, 1999, 22(2): 148-149. (in Chinese)
[24] YANG Hui-bin, PAN Xiao-lin, YU Hai-yan. Dissolution kinetics and mechanism of gibbsitic bauxite and pure gibbsite in sodium hydroxide solution under atmospheric pressure [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4151-4159.
[25] WHITTINGTON B I, CARDILE C M. The chemistry of tricalcium aluminate hexahydrate relating to the Bayer industry [J]. International Journal of Mineral Processing, 1996, 48: 21-38.
[26] SALIMI R, VAUGHAN J, PENG H. Solubility of tricalcium aluminate in synthetic spent Bayer liquor [J]. Industrial & Engineering Chemistry Research, 2014, 53: 17499-17505.
张佰永1,2,潘晓林1,王江洲1,于海燕1,涂赣峰1
1. 东北大学 冶金学院,沈阳 110819;
2. 沈阳铝镁设计研究院有限公司,沈阳 110001
摘 要:基于草酸盐石灰苛化法研究水合铝酸三钙和草酸钙分别在稀铝酸钠溶液和草酸钠溶液中的反应动力学和机理,并获得铝酸钠溶液去除草酸盐的最佳条件及其去除机理。水合铝酸三钙和草酸钙的生成分别受化学反应控制和内扩散控制,分别计算其反应速率方程和表观活化能。在含草酸盐的稀铝酸钠溶液中能够形成具有空间交错片状结构的水铝钙石相,其通过吸附大幅度降低溶液中草酸盐杂质。随着反应时间的延长,生成的草酸钙能够转化为水合铝酸三钙。在反应温度为50 °C、搅拌速度为200 r/min下,草酸盐苛化效率达到90%以上,氧化铝损失率在31%以下。
关键词:拜耳法;石灰苛化;草酸盐;动力学;铝酸钠溶液
(Edited by Xiang-qun LI)
Foundation item: Project (2018YFC1901903) supported by the National Key R&D Program of China; Projects (51774079, 51674075) supported by the National Natural Science Foundation of China; Project (N182508026) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: Xiao-lin PAN, Tel: +86-24-83686460, E-mail: panxl@smm.neu.edu.cn; Gan-feng TU, E-mail: tugf@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(19)65038-7

