DOI:10.19476/j.ysxb.1004.0609.2018.06.15
稀土矿浮选中Ce3+对萤石的活化作用机理
王介良1, 2,曹钊1,王建英2,张雪峰2,李解2,冯宏杰1
(1. 内蒙古科技大学 矿业研究院,包头 014010;
2. 内蒙古科技大学 内蒙古自治区白云鄂博矿多金属资源综合利用重点实验室,包头 014010)
摘 要:通过纯矿物浮选、Zeta 电位测试、溶液化学计算、红外光谱分析及XPS测试,研究以羟肟酸为捕收剂浮选稀土时Ce3+离子对萤石的活化作用机理。结果表明:矿浆中Ce3+离子浓度低于羟肟酸时在pH 6~12对萤石都具有活化作用,其活化机理为Ce3+离子吸附提高萤石表面的正电性,增强萤石表面与阴离子羟肟酸捕收剂的静电吸附作用,且在萤石表面形成F-Ce(OH)2/Ce(OH)+活性位点。红外光谱和XPS测试表明,无Ce3+离子活化时,辛基羟肟酸在萤石表面主要生成不稳定的四元环络合物—C—O—Ca—N—,羟肟酸与Ca2+离子结合能小,直接在萤石表面的吸附作用弱;被Ce3+离子活化后,辛基羟肟酸与萤石表面Ce3+离子反应生成稳定的五元环络合物—C= O—Ce—O—N—,羟肟酸与Ce3+离子结合能大,加强羟肟酸在萤石表面的吸附作用,从而使萤石被活化。
关键词:稀土;浮选;Ce3+离子;萤石;活化
文章编号:1004-0609(2018)-06-1196-08 中图分类号:TD923 文献标志码:A
稀土素有工业维生素之美称[1],其资源加工利用受到国内外学者广泛关注。浮选过程中萤石等含Ca脉石矿物难被抑制是稀土浮选生产面临的主要技术难题,主要原因是稀土矿物和含Ca脉石矿物萤石等均为半可溶性盐类矿物,在磨矿中易溶解产生大量的难免金属离子,难免金属离子在萤石等含Ca脉石矿物表面吸附会使其浮选受到活化[2-6]。已有研究表明,Cu2+离子、Fe3+离子会活化萤石浮选[7]。
在矿物浮选过程中,难免金属离子对矿物浮选的影响国内外学者已进行了大量研究。高跃升等[8]总结了各种金属离子对矿物浮选行为的影响规律及作用机理;卢佳等[9]研究表明,Ca2+离子可抑制十二胺对红柱石的浮选;曹钊等[10]研究表明,蛇纹石易受矿浆中Cu2+、Ni2+离子水解的金属氢氧化物沉淀活化;FORNASIERO等[11]研究发现,蛇纹石和绿泥石以及石英能够被Cu2+、Ni2+离子活化在pH 7~10范围内被黄药浮选;SHACKLETON等[12]研究表明,镍黄铁矿浮选矿浆中辉石易被Cu2+、Ni2+离子意外活化而上浮;高玉德等[13]研究认为Pb2+离子可活化苯甲羟肟酸对黑钨矿的浮选;HU等[14-15]研究表明,Pb2+离子与苯甲羟肟酸预先混合形成新型络合物捕收剂可活化白钨矿、锡石的浮选。此外,Pb2+离子对锂云母、石英[16]、锡石[17]、异极矿[18]也具有活化作用。
Ce3+离子是稀土矿浮选过程中难免金属离子,其在稀土矿浮选过程中对脉石矿物的活化作用研究鲜见报道。研究Ce3+离子对含Ca脉石矿物的活化作用及其机理,对解决稀土矿与含Ca脉石矿物分离的难题具有重要意义。基于此,本文作者通过萤石纯矿物浮选、Zeta电位测试、红外光谱测试、溶液化学计算以及X射线光电子能谱(XPS)测试等手段,研究稀土矿浮选中Ce3+离子对萤石的活化作用机制。
1 实验
1.1 试样制备
萤石矿取自山东临朐,矿石经手选除杂、破碎和陶瓷球磨机磨矿后,筛分获得38~74 μm粒级样品作为单矿物试验用纯矿物,萤石纯度在99%以上。
1.2 主要试剂
试验所用辛基羟肟酸(OHA)、NaOH、HCl和CeCl3 等试剂均为分析纯,实验用水为去离子水。
1.3 实验方法
1.3.1 浮选实验
萤石浮选实验在XFGCII-35型试验室用充气挂槽浮选机中进行,叶轮转速1992 r/min,每次称取2.00 g试验矿样加入到40 mL浮选槽中,加30 mL去离子水,浮选温度为室温。依次加入CeCl3搅拌调浆2 min,pH调整剂HCl或NaOH搅拌调浆2 min,捕收剂辛基羟肟酸搅拌调浆3 min,测量浮选前pH作为试验pH值,然后浮选刮泡4 min。对泡沫产品和槽内产品分别过滤,干燥称量,计算浮选回收率。试验流程如图 1 所示。

图1 萤石浮选实验流程
Fig. 1 Flowsheet of fluorite flotation experiment
1.3.2 表面电位测试
表面电位测试采用Brookhaven ZetaPlus Analyzer分析仪。将纯矿物样用玛瑙研钵研磨至粒径小于5 μm,每次精确称取50 mg置于小烧杯中,用去离子水配成50 mL的矿浆,用HCl或NaOH调节pH值,按照浮选条件依次加入一定浓度的浮选药剂,磁力搅拌器上搅拌5 min,取上述样品加入样品池,在Zetaplus Zeta电位测定仪上测量,每个样品重复测量3次,然后取平均值作为测试结果。
1.3.3 红外光谱(FTIR)测定
红外光谱测定采用溴化钾压片法在Bruker VERTEX 70型傅里叶变换红外光谱仪上进行。将纯矿物用玛瑙研钵研磨至粒径小于5 μm,每次称取1 g置于小烧杯中,用HCl或NaOH调节pH值至10,按照浮选条件依次加入一定浓度药剂,磁力搅拌器上搅拌10 min,过滤、同样pH值的去离子水洗涤样品两次,50 ℃下真空干燥后进行红外光谱测定。
1.3.4 XPS测试
XPS测试采用Thermo Scientific ESCALAB 250Xi 型X射线光电子能谱仪。称取1 g萤石置于小烧杯中,用HCl或NaOH调节pH值至10,按照浮选条件依次加入一定浓度的浮选药剂,磁力搅拌器上搅拌8 min,过滤、同样pH值的去离子水洗涤矿物两次,50 ℃下真空干燥后进行XPS测试。XPS测试条件为:单色化Al Kα 射线光源,hν=1486.6 eV,500 μm光斑面积,真空度为5×10-8 Pa,C 1s矫正值284.8 eV;全谱扫描结合能范围为0~1300 eV,步长1.0 eV,通过能为100 eV;N 1S高分辨谱通过能为30 eV,步长0.05 eV。采用CasaXPS进行谱峰分析和分峰拟合。
2 结果与讨论
2.1 萤石的浮选行为
图2所示为辛基羟肟酸OHA为捕收剂时,Ce3+离子对萤石浮选回收率的影响。其中,曲线a、b、c、d分别表示羟肟酸OHA浓度为5×10-5 mol/L,添加Ce3+离子浓度为0、5×10-5、1×10-4、2×10-4 mol/L时萤石的浮选回收率曲线。由图2可知,当辛基羟肟酸浓度为5×10-5 mol/L,在添加或者不添加Ce3+离子的情况下,随pH值增大,萤石浮选回收率均先增大后降低,在pH=10时,萤石浮选回收率达到最大值。因此,萤石浮选最佳pH值为pH=10。当不添加Ce3+离子时,萤石浮选回收率最大值为42.35%(见图2(a));当添加Ce3+离子且矿浆中Ce3+离子浓度为5×10-5 mol/L时,在实验pH值范围内,Ce3+离子明显活化萤石浮选,其回收率最大值达到83.67%(见图2(b));当矿浆中Ce3+离子浓度为1×10-4 mol/L时,萤石浮选回收率最大值为82.14%(见图2(c)),较Ce3+离子浓度为5×10-5 mol/L时萤石浮选最大回收率略有下降;当矿浆中Ce3+离子浓度达到2×10-4 mol/L时,萤石浮选回收率最大值降到44.39%(见图2(d)),此浓度下Ce3+离子抑制了萤石浮选。以上实验结果表明,Ce3+离子浓度低时对萤石浮选具有活化作用,Ce3+离子浓度高时对萤石浮选具有抑制作用。主要是因为Ce3+离子浓度高时,矿浆中过量的Ce3+离子会消耗辛基羟肟酸,降低矿浆中辛基羟肟酸浓度,导致萤石浮选回收率下降,从而表现出对萤石浮选的抑制作用。
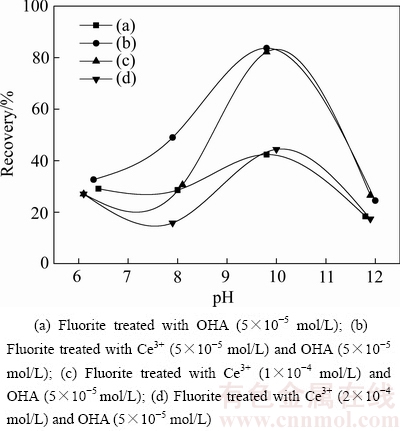
图2 不同pH下Ce3+对萤石纯矿物浮选回收率的影响
Fig. 2 Effect of Ce3+ on recovery of fluorite at different pH values
2.2 机理分析
为探讨Ce3+离子对萤石浮选的活化作用机理,进行了溶液化学计算和Zeta电位、红外光谱以及XPS测试与分析。
2.2.1 溶液化学计算
矿浆中金属离子的水化平衡,是研究其在浮选中作用的基础。溶液中各组分浓度可通过溶液平衡关系求出,以此绘制lg c-pH图,结合浮选实验结果,确定起活化作用的优势组分[7]。
弱酸根阴离子在矿物表面的吸附能力与其解离常数有关。图3所示为辛基羟肟酸OHA用量为5×10-5 mol/L时的lg c-pH图[19],由图3可知,随着pH的升高,溶液中羟肟酸阴离子含量增加,结合图2可知,萤石的浮选回收率也随着升高。因此,羟肟酸阴离子是决定萤石上浮的主要活性成分。

图3 辛基羟肟酸溶液体系lg c-pH图 (辛基羟肟酸浓度为5×10-5 mol/L)
Fig. 3 lg c-pH diagram of OHA solution system (c(OHA)= 5×10-5 mol/L)
FORNASIERO、FUERSTENAU等[11, 20]认为,矿浆中金属离水解反应生成的各种羟基络合物,吸附在氧化矿表面从而活化氧化矿的浮选;王淀佐等[7]认为金属氢氧化物表面沉淀也是金属离子在氧化矿表面吸附并起活化作用的有效组分。图4所示为矿浆中Ce3+离子浓度为5×10-5 mol/L时,Ce3+离子水解反应组分lg c-pH图,由图4结合Ce3+离子活化后萤石浮选行为分析可知,在pH=6~7时,矿浆中Ce主要以Ce3+存在,此时萤石受到活化作用较弱;在pH=11~12时,矿浆中Ce主要以Ce(OH)3(s)存在,此时萤石受到活化作用也较弱;而在pH=7~11范围内,羟基络合物CeOH2+、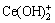 含量较大,在此pH范围内萤石浮选受到强烈活化。结合图3中辛基羟肟酸优势组分图分析可知,羟基络合物CeOH2+、
含量较大,在此pH范围内萤石浮选受到强烈活化。结合图3中辛基羟肟酸优势组分图分析可知,羟基络合物CeOH2+、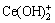 是活化萤石浮选的主要组分。
是活化萤石浮选的主要组分。
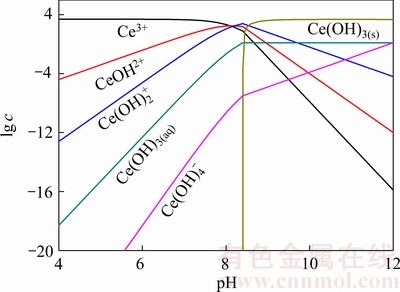
图4 Ce3+溶液的lg c-pH图 (Ce3+浓度为5×10-5 mol/L)
Fig. 4 lg c-pH diagram of Ce3+ solution (c(Ce3+)=5×10-5 mol/L)
2.2.2 Zeta电位测试
矿物在溶液中由于表面正、负离子的结合能及受水化作用强度不同会发生非等当量解离,此外当向溶液中加入浮选药剂时,药剂在水中解离,会在矿物表面发生吸附,这些因素都会影响矿物Zeta电位的变化。因此,进行Zeta电位测定可进一步揭示药剂与矿物的作用机理[7]。
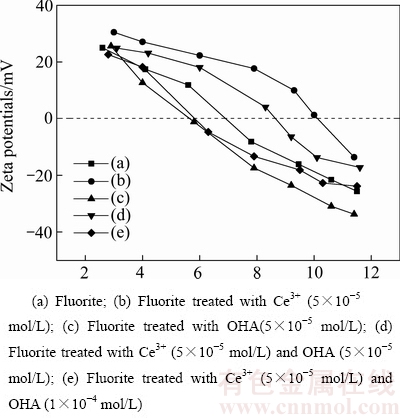
图5 Ce3+离子及辛基羟肟酸对萤石Zeta电位的影响
Fig. 5 Effect of Ce3+ ions and OHA on Zeta potential of fluorite
图5所示为Ce3+离子和辛基羟肟酸对萤石Zeta电位的影响曲线。其中,曲线(a)、(b)、(c)、(d)、(e)分别表示萤石、5×10-5 mol/L浓度Ce3+离子活化萤石、5×10-5 mol/L浓度羟肟酸OHA与萤石作用、5×10-5 mol/L浓度羟肟酸OHA与Ce3+离子活化后萤石作用、1×10-4 mol/L浓度羟肟酸OHA与Ce3+离子活化后萤石作用时萤石的Zeta电位的曲线图。由图5可知,萤石与辛基羟肟酸作用后动电位整体向负值方向偏移,等电点由pH=6.8(见图5中曲线(a))降低至5.6(见图5中曲线(c)),说明带负电荷的辛基羟肟酸根离子吸附在萤石表面,增加萤石表面负电性;当矿浆中加入Ce3+离子时,萤石动电位整体向正值方向偏移,等电点由pH=6.8升高至10.2(见图5中曲线(b)),说明Ce3+离子吸附在萤石表面,增加萤石表面正电性;当矿浆中依次加入Ce3+离子和辛基羟肟酸时,萤石动电位比在矿浆中只加入Ce3+离子时向负值方向偏移(见图5中曲线(d)),且加入辛基羟肟酸浓度越大,萤石动电位向负值方向偏移越大(见图5中曲线(e)),说明辛基羟肟酸浓度越大,其在萤石表面的吸附量越大。
结合Ce3+离子存在时萤石浮选行为分析可知,Ce3+离子吸附在萤石表面后增强阴离子捕收剂辛基羟肟酸在萤石表面的静电吸附力,强化羟肟酸在萤石表面的吸附,达到活化的效果。
2.2.3 FTIR测试
图6所示为辛基羟肟酸OHA及辛基羟肟酸与Ce3+离子在pH=10时混合生成的Ce-OHA沉淀的红外光谱图。由图6可知,辛基羟肟酸红外光谱(见图6中曲线(a))中1566.19 cm-1处为—C=O伸缩振动峰,1664.56 cm-1、1625.98 cm-1处为—C=N伸缩振动峰,3257.75 cm-1处为—N—H和—O—H叠加伸缩振动峰,2956.85cm-1、2916.35 cm-1、2846.91 cm-1处为CH2—/—CH3伸缩振动峰[3, 21]; Ce-OHA沉淀红外光谱(见图6中曲线(b))图中—C=O、—C=N伸缩振动峰分别红移至1525.68 cm-1、1600.9 cm-1处,主要是因为羟肟酸OHA与Ce3+络合造成—C=O、—C=N双键上电子云密度向Ce3+偏移,使得双键键能减弱。
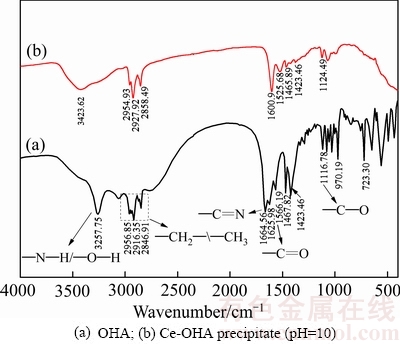
图6 辛基羟肟酸及辛基羟肟酸-铈沉淀的红外光谱
Fig. 6 FTIR spectra of OHA and Ce-OHA Precipitate
图7(a)、(b)、(c)、(d)所示分别为萤石、萤石直接与辛基羟肟酸OHA作用、萤石被Ce3+活化后再与辛基羟肟酸作用以及Ce-OHA络合沉淀物的红外光谱。由图7可知,辛基羟肟酸直接在萤石表面作用时(见图7(b)),只出现较弱的—C=N双键伸缩振动峰(1631 cm-1处),且波数发生红移(-32.79 cm-1)较小,同时3257.75 cm-1处—N—H和—O—H叠加伸缩振动峰没有出现,说明羟肟酸在萤石表面吸附较弱,只能生成不稳定的四元环络合物;Ce-OHA络合沉淀物(见图7(d))在1525.68 cm-1和1600.9 cm-1处震动峰分别为 —C=O和—C=N伸缩振动峰;辛基羟肟酸在Ce3+离子活化后萤石表面作用(见图7(c))时,出现较强的—C=O(1516.04 cm-1处)和—C=N(1608.62 cm-1处)伸缩振动峰,且—C=N伸缩振动峰红移(55.94 cm-1)波数大于辛基羟肟酸直接在萤石表面作用时的红移波数,同时3257.75 cm-1处—N—H和—O—H叠加伸缩振动峰没有出现,说明辛基羟肟酸在Ce3+离子活化后萤石表面吸附较强并生成了稳定的五元环络合物[21]。
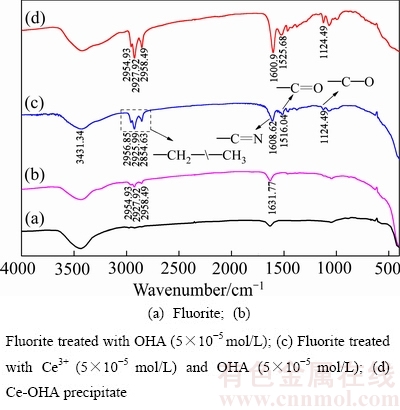
图7 萤石、萤石与OHA作用及萤石被Ce3+活化后与OHA作用的红外光谱
Fig. 7 FTIR spectra of fluorite, fluorite treated with OHA and Ce3+ activated fluorite treated with OHA
2.2.4 XPS测试
X射线光电子能谱(XPS)一种重要的表面分析方法,可通过对内层电子结合能的化学位移精确测量来确定元素的化学状态、化学键和电荷分布,对元素进行定性分析、定量分析;同时可以进行包括化学组成或元素组成、原子价态、表面能态分布、表面电子的电子云分布和能级结构等在内的固体表面分析[22]。
为进一步分析Ce3+离子和辛基羟肟酸在萤石表面作用方式,进行了萤石、萤石与Ce3+离子作用、萤石与羟肟酸作用及萤石经Ce3+离子活化后与羟肟酸作用的XPS能谱检测。
图8(a)、(b)、(c)、(d)所示分别为萤石、萤石与Ce3+离子作用后、萤石与羟肟酸作用后及萤石经Ce3+离子活化再与羟肟酸作用后的XPS能谱。与图8(a)中萤石的XPS峰相比,Ce3+离子活化后萤石表面出现Ce 3d XPS峰,即Ce3+在萤石表面发生吸附(见图8(b));萤石直接与羟肟酸作用后表面仅出现较弱的N 1s XPS峰(见图8(c)),萤石经Ce3+活化后再与羟肟酸作用表面会出现较强的N 1s XPS峰(见图8(d)),说明Ce3+活化增强了羟肟酸在萤石表面的吸附作用。XPS能谱检测结果与萤石浮选实验及红外光谱研究结果相一致。
表1所列为萤石、萤石与Ce3+离子作用、萤石与羟肟酸作用及萤石经Ce3+离子活化后与羟肟酸作用后的元素原子轨道结合能。由表1可知,萤石与Ce3+离子作用后,萤石表面F 1s 的结合能偏移0.35 eV,萤石表面存在Ce的化学吸附,并形成F—Ce(OH)2或者F—Ce(OH)+键;萤石与羟肟酸作用后,Ca和F元素的结合能偏移均不大于0.1 eV,结合图8分析可知,萤石与羟肟酸直接作用时,萤石表面存在羟肟酸的化学吸附,但吸附作用较弱;萤石经Ce3+离子活化后再与羟肟酸作用,萤石表面Ce 3d 结合能偏移1.25 eV,说明羟肟酸与萤石表面吸附的Ce3+离子发生化学吸附,N 1s 的结合能较萤石与羟肟酸直接作用的结合能偏移0.4 eV,说明Ce3+离子强化羟肟酸在萤石表面的化学吸附的方式是羟肟酸与吸附在萤石表面的Ce3+相结合。此外,辛基羟肟酸铈沉淀的溶度积(1×10-21.9)小于辛基羟肟酸钙溶度积(1×10-5.22),表明萤石表面经Ce3+活化后,更有利于羟肟酸捕收剂在其表面产生疏水性沉淀[7],从而对其浮选产生活化。

图8 萤石、萤石与Ce3+作用、萤石与OHA作用及萤石被Ce3+活化后与OHA作用的XPS全谱
Fig. 8 XPS survey scan of fluorite, fluorite treated with Ce3+, fluorite treated with OHA and Ce3+ activated fluorite treated with OHA
表1 萤石、萤石与Ce3+作用、萤石与OHA作用及萤石被Ce3+活化后与OHA作用后元素原子轨道结合能
Table 1 Binding energies of elements of fluorite, fluorite treated with Ce3+, fluorite treated with OHA and Ce3+ activated fluorite treated with OHA
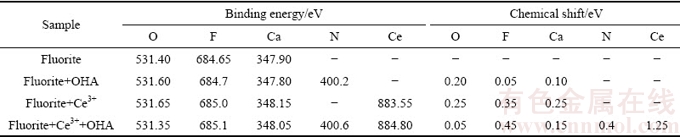
图9所示为Ce3+离子活化前后萤石与辛基羟肟酸作用的N 1s XPS高分辨谱。由图9可知,萤石不与辛基羟肟酸作用时表面未出现N 1s XPS峰(见图9(a)),萤石直接与羟肟酸作用后表面出现了400.2 eV(为羟肟酸金属五元环N 1s XPS峰)和398.5 eV(为羟肟酸金属四元环N 1s XPS峰)两处峰[21, 23](见图9(b)),400.2 eV处峰弱于398.5 eV处峰,表明萤石直接与羟肟酸作用后产物以不稳定的四元环络合物为主);而经Ce3+活化后萤石再与羟肟酸作用后N 1s XPS峰(见图9(c))更强、面积更大,且表面400.6 eV峰(五元环N 1s)强于398.9 eV峰(四元环N 1s),表明Ce3+活化使羟肟酸在萤石表面生成了更稳定的五元环络合物,增强了捕收剂在萤石表面的吸附作用。
根据以上测试分析,可预测羟肟酸在Ce3+活化前后的萤石{110}面暴露面[24-25]的吸附过程如图10~11所示。由图10可知,辛基羟肟酸直接与萤石作用时,与萤石表面Ca生成不稳定四元环络合物—C—O—Ca—N—(见图10(b));由图11可知,辛基羟肟酸与经Ce3+离子活化的萤石作用时,辛基羟肟酸不但会直接与萤石表面的Ca反应生成不稳定四元环络合物—C—O—Ca—N—,而且会与萤石表面吸附的Ce3+离子反应,其活化过程如下:1) 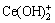 、Ce(OH)2+与萤石表面F—Ca断键露出的F-反应生成F—Ce(OH)2或者F—Ce(OH)+活性位点(见图11(b));2) 辛基羟肟酸与萤石表面活性位点反应生成稳定的五元环络合物—C=O—Ce—O—N—(见图11(c)),使萤石疏水性增大,活化萤石浮选。
、Ce(OH)2+与萤石表面F—Ca断键露出的F-反应生成F—Ce(OH)2或者F—Ce(OH)+活性位点(见图11(b));2) 辛基羟肟酸与萤石表面活性位点反应生成稳定的五元环络合物—C=O—Ce—O—N—(见图11(c)),使萤石疏水性增大,活化萤石浮选。

图9 Ce3+离子活化前后萤石与辛基羟肟酸作用的N 1s XPS高分辨谱
Fig. 9 N1s XPS high-resolution scan of OHA treated with fluorite before/after activated by Ce3+
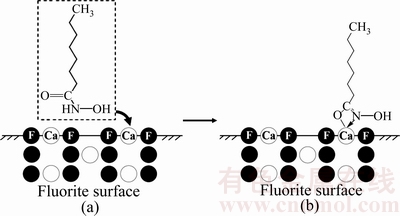
图10 辛基羟肟酸在萤石表面的吸附过程
Fig. 10 Adsorption process of OHA on fluorite surface

图11 辛基羟肟酸在Ce3+离子活化后萤石表面的吸附过程
Fig. 11 Adsorption process of OHA on fluorite surface activated by Ce3+
3 结论
1) 当辛基羟肟酸浓度为5×10-5 mol/L、矿浆中Ce3+离子浓度小于5×10-5 mol/L时,在pH 6~12之间对萤石浮选具有活化作用;Ce3+离子浓度≥2×10-4 mol/L时,矿浆中过量的Ce3+离子会消耗辛基羟肟酸,从而降低萤石浮选回收率。
2) Ce3+离子活化萤石浮选的机理是:矿浆中羟基络合物Ce(OH)2+、Ce(OH)2+与萤石表面F—Ca断键露出的F-反应生成F—Ce(OH)2或者F—Ce(OH)+活性位点,增强羟肟酸在萤石表面的吸附能力,从而活化萤石浮选。
3) 无Ce3+离子活化时,辛基羟肟酸与萤石表面主要生成不稳定四元环络合物—C—O—Ca—N—,羟肟酸与Ca结合能小,吸附作用弱;萤石被Ce3+离子活化后,辛基羟肟酸在萤石表面生成物以稳定五元环络合物—C=O—Ce—O—N—为主,羟肟酸与Ce3+离子结合能大,增强了羟肟酸在萤石表面的吸附能力,从而使其浮选受到活化。
REFERENCES
[1] 张培善, 陶克捷. 中国稀土矿物学[M]. 北京: 科学出版社, 1998: 3-4.
ZHANG Pei-shan, TAO Ke-jie. Chinese rare earth mineralogy[M]. Beijing: Science Press, 1998: 3-4.
[2] FUERSTENAU D W, PRADIP, HERRERA-URBINA R. The surface chemistry of bastnaesite, barite and calcite in aqueous carbonate solutions[J]. Colloids and Surfaces, 1992, 68: 95-102.
[3] ZHANG W, HONAKER R Q, GROPPO J G. Flotation of monazite in the presence of calcite part Ⅰ: Calcium ion effects on the adsorption of hydroxamic acid[J]. Minerals Engineering, 2017, 100: 40-48.
[4] ZHANG X, DU H, WANG X, MILLER J D. Surface chemistry considerations in the flotation of rare-earth and other semisoluble salt minerals[J]. Minerals & Metallurgical Processing, 2013, 30(1): 24-37.
[5] 卢烁十, 孙传尧. 盐类矿物浮选现状[J]. 矿冶, 2007, 16(3): 1-4.
LU Shuo-shi, SUN Chuan-yao. Review of salt minerals flotation[J]. Mining and Metallurgy, 2007, 16(3): 1-4.
[6] 王淀佐, 胡岳华. 浮选溶液化学[M]. 长沙: 湖南科学技术出版社, 1988: 132-148, 336.
WANG Dian-zuo, HU Yue-hua. Solution chemistry of flotation[M]. Changsha: Hunan Science and Technology Press, 1988: 132-148, 336.
[7] 张 波, 李 解, 张雪峰, 曹 钊, 王维大, 韩 华. Cu2+, Fe3+对萤石浮选的活化作用机制[J]. 稀有金属, 2016, 40(9): 963-968.
ZHANG Bo, LI Jie, ZHANG Xue-feng, CAO Zhao, WANG Wei-Da, HAN Hua. Activation and mechanism of Cu2+ and Fe3+ in flotation system of fluorite ore[J]. Chinese Journal of Rare Metals, 2016, 40(9): 963-968.
[8] 高跃升, 高志勇, 孙 伟. 金属离子对矿物浮选行为的影响及机理研究进展[J]. 中国有色金属学报, 2017, 27(4): 859-868.
GAO Yue-sheng, GAO Zhi-yong, SUN Wei. Research progress of influence of metal ions on mineral flotation behavior and underlying mechanism[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(4): 859-868.
[9] 卢 佳, 高惠民, 金俊勋, 岑对对, 任子杰. 钙离子对红柱石浮选的影响及作用机理[J]. 中国有色金属学报, 2016, 26(6): 1311-1315.
LU Jia, GAO Hui-min, JIN Jun-xun, CEN Dui-dui, REN Zi-jie. Effect and mechanism of calcium ion on flotation of andalusite[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(6): 1311-1315.
[10] 曹 钊, 张亚辉, 孙传尧, 曹永丹. 铜镍硫化矿浮选中Cu(II)和Ni(II)离子对蛇纹石的活化机理[J]. 中国有色金属学报, 2014, 24(2): 506-510.
CAO Zhao, ZHANG Ya-hui, SUN Chuan-yao, CAO Yong-dan. Activation mechanism of serpentine by Cu(Ⅱ) and Ni(Ⅱ) ions in copper-nickel sulfide ore flotation[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(2): 506-510.
[11] FORNASIERO D, RALSTON J. Cu(Ⅱ) and Ni(Ⅱ) activation in the flotation of quartz, lizardite and chlorite[J]. International Journal of Mineral Processing, 2005, 76(1): 75-81.
[12] SHACKLETON N J, MALYSIAK V, OCONNOR C T. The use of amine complexes in managing inadvertent activation of pyroxene in a pentlandite-pyroxene flotation system[J]. Minerals Engineering, 2003, 16(9): 849-856.
[13] 高玉德, 钟传刚, 邱显扬, 冯其明, 万 丽. 苯甲羟肟酸浮选黑钨矿体系中铅离子的活化作用机理[J]. 中国有色金属学报, 2016, 26(9): 1999-2006.
GAO Yu-de, ZHONG Chuan-gang, QIU Xian-yang, FENG Qi-ming, WAN Li. Activation mechanism of Pb2+ in flotation of wolframite with benzohydroxamic acid as collector[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(9): 1999-2006.
[14] HAN H, HU Y, SUN W, LI X, CAO C, LIU R, YUE T, MENG X, GUO Y, WANG J, GAO Z, CHEN P, HUANG W, LIU J, XIE J, CHEN Y. Fatty acid flotation versus BHA flotation of tungsten minerals and their performance in flotation practice[J]. International Journal of Mineral Processing, 2017, 159: 22-29.
[15] TIAN M, HU Y, SUN W, LIU R. Study on the mechanism and application of a novel collector-complexes in cassiterite flotation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017, 522: 635-641.
[16] 孙传尧, 贾木欣. 用油酸钠作捕收剂几种硅酸盐矿物浮游性的分析[J]. 有色金属, 2001, 53(4): 57-61.
SUN Chuan-yao, JIA Mu-xin. Flotability of some silicate minerals with sodium oleate as collector[J]. Nonferrous Metals, 2001, 53(4): 57-61.
[17] FENG Qi-cheng, ZHAO Wen-juan, WEN Shu-ming, CAO Qin-bo. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector[J]. Separation and Purification Technology, 2017, 178: 193-199.
[18] LIU Cheng, FENG Qi-ming, ZHANG Guo-fan, MA Wan-kun, MENG Qing-you, CHEN Yan-fei. Effects of lead ions on the flotation of hemimorphite using sodium oleate[J]. Minerals Engineering, 2016, 89: 163-167.
[19] MENG Qing-you, FENG Qi-ming, SHI Qing, OU Le-ming. Studies on interaction mechanism of fine wolframite with octyl hydroxamic acid[J]. Minerals Engineering, 2015, 79: 133-138.
[20] FUERSTENAU D, RAGHAVAN S. Flotation[M]. New York: AIME, 1976.
[21] 饶金山, 何晓娟, 罗传胜, 陈志强. 辛基羟肟酸浮选氟碳铈矿机制研究[J]. 中国稀土学报, 2015, 33(3): 370-377.
RAO Jin-shan, HE Xiao-juan, LUO Chuan-sheng, CHEN Zhi-qiang. Flotation mechanism of octyl hydroximic acid on bastnaesite[J]. Journal of the Chinese Society of Rare Earths, 2015, 33(3): 370-377.
[22] 张 英, 胡岳华, 王毓华, 文书明. 硅酸钠对含钙矿物浮选行为的影响及作用机理[J]. 中国有色金属学报, 2014, 24(9): 2366-2372.
ZHANG Ying, HU Yue-hua, WANG Yu-hua, WEN Shu-ming. Effects of sodium silicate on flotation behavior of calcium-bearing minerals and its mechanism[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2366-2372.
[23] WAGNER C D, MUILENBERG G E. Hand book of X-ray photoelectron spectroscopy[M]. Eden Prairie Minnesota: Perkin-Elmer Corporation, 1979: 38.
[24] de LEEUW N H, COOPER T G. A computational study of the surface structure and reactivity of calcium fluoride[J]. Journal of Materials Chemistry, 2003, 13(1): 93-101.
[25] 高跃升, 高志勇, 孙 伟. 萤石表面性质各向异性研究及进展[J]. 中国有色金属学报, 2016, 26(2): 415-422.
GAO Yue-sheng, GAO Zhi-yong, SUN Wei. A review of anisotropic surface properties of fluorite[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(2): 415-422.
Activation mechanism of Ce3+ on fluorite in rare earth ore flotation
WANG Jie-liang1, 2, CAO Zhao1, WANG Jian-ying2, ZHANG Xue-feng2, LI Jie2, FENG Hong-jie1
(1. Institute of Mining Engineering, Inner Mongolia University of Science and Technology, Baotou 014010, China;
2. Key Laboratory of Integrated Exploitation of Bayan Obo Multi-Metal Resources, Inner Mongolia University of Science and Technology, Baotou 014010, China)
Abstract: The activation mechanism of Ce3+ on fluorite using octanohydroxamic acid (OHA) as rare earth flotation collector was studied by pure mineral floatation experiment, Zeta potential measurement, solution chemistry analysis, infrared( IR) spectra and XPS measurements. The results show that fluorite can be activated at pH 6-12 when the concentration of Ce3+ is lower than that of OHA, the activation mechanism is that the positive potential of fluorite is improved by adsorbing of Ce3+onto its surface, thus enhancing the electrostatic adsorption of anionic hydroxmic acid collector on fluorite, and moreover F-Ce(OH)2/Ce(OH)+ active site can be formed on the surface of fluorite. IR spectra and XPS measurements show that an unstable four-member ring chelate complex —C—O—Ca—N— is generated when OHA reacts with fluorite without Ce3+ activation, the binding energy of OHA with Ca2+ is low, which leads to a weak adsorption of OHA onto fluorite. A stable five-member ring chelate complex —C=O—Ce—O—N— forms when OHA reacts with Ce3+ activated fluorite, the binding energy of OHA with Ce3+ is high, which enhances the adsorption of OHA onto fluorite and thus activates the flotation of fluorite.
Key words: rare earth; flotation; Ce3+ ion; fluorite; activation
Foundation item: (Project(2014MS0521) supported by the Natural Science Foundation of Inner Mongolia, China; Project(414060901) supported by the Science and Technology Major Projects of Inner Mongolia, China
Received date: 2017-04-07; Accepted date: 2017-07-10
Corresponding author: ZHANG Xue-feng; Tel: +86-13190691928; E-mail:xuefeng367@163.com
(编辑 龙怀中)
基金项目:内蒙古自然科学基金资助项目(2014MS0521);内蒙古科技重大专项资助项目(414060901)
收稿日期:2017-04-07;修订日期:2017-07-10
通信作者:张雪峰,教授,博士;电话:13190691928;E-mail: xuefeng367@163.com