菱镁矿制备轻烧氧化镁及其水化动力学研究
刘欣伟1,冯雅丽1,李浩然2,张萍1,汪平1
(1. 北京科技大学 土木与环境工程学院,北京 100083;
2. 中国科学院 过程工程研究所生化工程国家重点实验室,北京 100190)
摘要:以菱镁矿为原料,制备轻烧氧化镁,并对轻烧氧化镁的水化动力学进行研究,考察水化温度,氧化镁质量浓度和颗粒度对水化过程的影响。研究结果表明:煅烧温度和保温时间对氧化镁活性影响要比颗粒度的大,最佳煅烧条件是温度为750 ℃,煅烧时间为1.5 h,颗粒度为1 mm;氧化镁的水化反应过程为内扩散控制类型,表观反应活化能为23.78 kJ/mol,在此基础上,用MATLAB做多元线性归回分析,得到表观反应速率常数关于水化温度、氧化镁浓度和颗粒度的经验表达式。
关键词:活性氧化镁;水化率;水化反应;动力学;内扩散控制
中图分类号:TQ132.2 文献标志码:A 文章编号:1672-7207(2011)12-3912-06
Preparation of light-burned magnesia from magnesite and its hydration kinetics
LIU Xin-wei1, FENG Ya-li1, LI Hao-ran2, ZHANG Ping1, WANG Ping1
(1. Civil and Environment Engineering School, University of Science and Technology Beijing, Beijing 100083, China;
2. State Key Laboratory of Biochemical Engineering, Institute of Process Engineering,
Chinese Academy of Science, Beijing 100190, China)
Abstract: Light-burned magnesia was prepared from magnesite ore, and the hydration dynamic of light-burned magnesia was investigated. The effects of temperature (θ), mass concentration of magnesia (ρ0) and particle size (r0) of magnesia on hydration kinetics were studied. The calcination tests show that calcination temperature (θ) and heating time (t) have greater influence than the particle size (r0) on the activity of magnesia. And the best calcination condition is that θ=750 ℃, t=1.5 h, and r0=1 mm. The hydration process of magnesia is controlled by intra-particle diffusion, and the apparent activation energy is 23.78 kJ/mol. Based on the theory model, an experiential formular including three parameters (θ, c0 and r0) is proposed through MATLAB multiple regression analysis.
Key words: active magnesium oxide; hydration rate; hydration reaction; dynamics; intra-particle diffusion control
高纯氧化镁指MgO质量分数大于98%的样品,高温下具有优良的耐碱性和电绝缘性,光透过性好,导热性高,热膨胀系数大,广泛用于电子、化工、陶瓷等行业[1-5]。目前制备高纯氧化镁的方法主要有海水石灰沉淀法、卤水热解法和菱镁矿煅烧法[6-7],海水石灰法工艺复杂,投资大,生产成本高;盐湖卤水热解法工艺过程中副产大量的氯化氢,腐蚀性大,回收利用较困难。菱镁矿煅烧法工艺在生产高纯氧化镁的过程中不会对环境造成污染,是理想的生产氧化镁的工艺。但目前在菱镁矿煅烧制备轻烧氧化镁和再水化、碳化法制备高纯氧化镁的工艺中,轻烧氧化镁活性和水化效率直接影响到碳化率和综合回收率。在以前的研究中,主要考察菱镁矿煅烧条件和水化温度对轻烧氧化镁水化率的影响[8-9]。在此,本文作者以菱镁矿为原料制备轻烧氧化镁,考察水化温度、氧化镁浓度和颗粒度对轻烧氧化镁水化动力学的影响,并建立表观反应速率常数关于水化温度、氧化镁浓度和颗粒度的经验表达式,以便为菱镁矿水化、碳化法制备高纯氧化镁提供理论依据。
1 实验部分
1.1 主要原料及实验设备
本项研究选用辽宁海城菱镁矿为原料,其化学组成(质量分数)见表1。
表1 菱镁矿的化学组成
Table 1 Chemical composition of magnesite %

主要实验设备为:KSW-5-12A型电炉温度控制器;数控超线恒温槽;AR1140电子天平;SHZ-D(III)循环水式真空泵;PH050电烘干炉。
1.2 实验方法及测试方法
1.2.1 轻烧氧化镁的制备
轻烧氧化镁由菱镁矿煅烧制得,分解过程为MgCO3→MgO+CO2;制备工艺为:菱镁矿鄂式破碎→筛分为一定粒度→一定煅烧温度和保温时间下煅烧→轻烧氧化。1Mass fraction%)⑵
1.2.2 轻烧氧化镁活性的测定
将一定颗粒度的菱镁矿分别在750,800和900 ℃下煅烧,冷却后用研钵磨细至粒度在75 μm以下。在80 ℃水浴中,采用柠檬酸活性法表征轻烧氧化镁的反应活性[10]。
1.2.3 水化反应
将轻烧氧化镁与水按不同的比例混合,用电动磁力搅拌器设定不同的转速,分别在30,40,50,60和70 ℃下进行恒温水浴,在不同温度下分别反应0.5,1.0,1.5,2.0和2.5 h后,再将水化后悬浮液抽滤,清洗,产物至于70 ℃烘箱中干燥至恒质量。
1.2.4 水化率测定
将干燥后的水化产物于500 ℃煅烧2 h,由下式得到水化率:
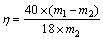 (1)
(1)
式中:η为水化率;m1为水化产物煅烧前质量;m2为水化产物煅烧后质量。
2 结果与讨论
2.1 活性氧化镁制备条件优化研究
选择适当的煅烧温度、保温时间和原矿粒度对菱镁矿进行煅烧,考察煅烧温度、保温时间以及原矿粒度对轻烧氧化镁活性的影响,用L9(34)正交设计表进行方差分析,研究各因素对氧化镁活性的影响,优化制备条件。氧化镁正交试验正交表如表2所示。不同条件下氧化镁活性见表3。采用方差分析法处理试验数据,结果见表4。
表2 因素水平确定表
Table 2 Determination of factors and levels

表3 氧化镁活性正交分析表
Table 3 Orthogonal analysis of magnesium oxide activity
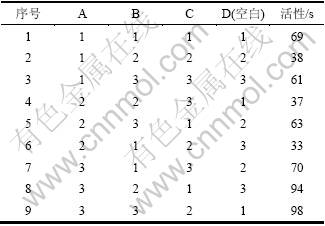
表4 氧化镁活性方差分析结果
Table 4 Variance analysis of magnesia activity
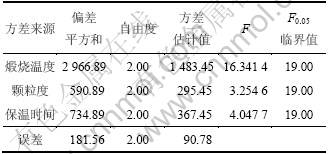
由表4可知:煅烧温度的偏差平方和最大,保温时间的次之,颗粒度的最小,因此,影响氧化镁活性的最主要因素为煅烧温度,其次为保温时间,颗粒度的影响较小。从理论上讲,菱镁矿的颗粒越小,热传导越快,菱镁矿分解越充分,轻烧氧化镁的活性越高。另外,菱镁矿是三方晶系,MgCO3受热分解过程是 [CO3]结构单元的位置上释放出1个CO2,留下1个O,使这些位置的化学组成发生变化,成为物化性能活化点。在700~800 ℃煅烧时,菱镁矿化学组成由MgCO3转变成MgO,价键由Mg—[CO3]转变为Mg—O,但晶体结构仍维持菱镁矿的结构,晶体结构的键角大、结构疏松、不规则,因而化学活性高;随着煅烧温度升高和保温时间延长,菱镁矿的分解反应基本结束,晶体结构调整和生长占主导地位,MgO晶体渐趋完善、晶粒尺寸变大,化学活性逐渐降低,但当煅烧温度较低,保温时间过短时,热量无法传到颗粒内部,颗粒内外温差较大,导致所谓“加生”现象[11-13],菱镁矿分解不完全,为保证轻烧料不欠烧也不过烧,并具有较高的活性,最佳煅烧温度应控制在750 ℃,煅烧时间为1.5 h,颗粒度为1 mm。
2.2 水化反应动力学研究
2.2.1 轻烧氧化镁水化动力学模型的确定
轻烧氧化镁的水化反应方程为:MgO+H2O→ Mg(OH)2,根据水化条件的不同,水化动力学可以分为化学反应控制、外扩散控制和内扩散控制3种类 型[14-18]。为了确定氧化镁水化动力学模型,首先研究搅拌速度对氧化镁水化率的影响,结果如图1所示。
在轻烧氧化镁水化过程中,当液膜扩散为控速步骤时,搅拌强度对水化率影响很大,通常可提高水化率40%~70%[19]。但从实验结果(图1)可知:搅拌强度对氧化镁的水化率影响不大,由此可判断水化反应控制步骤非外扩散控制。
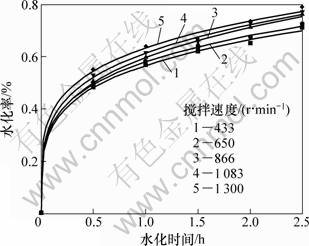
图1 搅拌速度对氧化镁水化率的影响
Fig.1 Effect of stirring speed on magnesia hydration rate
为了进一步判断氧化镁水化动力学模型,将实验数据代入不同的动力学方程拟合,不同温度下的实验数据与内扩散控制的动力学方程为:
g(α)=1-2/3α-(1-α)2/3=kt (2)
式中:g(α)代表水化率函数;α为水化率;k为表观反应速率常数;t为水化时间。拟合结果如图2所示。从图2可见:不同温度下各实验数据与内扩散控制动力学方程均较好地符合(R2>0.98),从而证实轻烧氧化镁的水化反应为内扩散控制类型。
阿累尼乌斯公式:
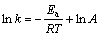 (3)
(3)
式中:T为热力学温度;Ea为反应活化能。将水化温度与水化速率常数之间的关系拟合(见图3),可求得表观反应活化能为23.78 kJ/ mol,根据化学反应与扩散控速的活化能判据: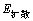 <25.12 kJ/mol<
<25.12 kJ/mol< ,可以进一步判定氧化镁的水化反应为内扩散控制类型。
,可以进一步判定氧化镁的水化反应为内扩散控制类型。
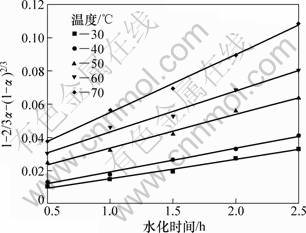
图2 不同温度下的水化动力学曲线
Fig.2 Hydration dynamic curves at different temperatures
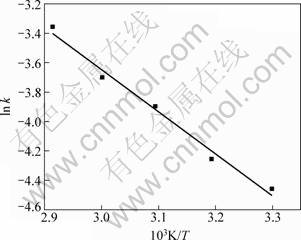
图3 反应速率常数与温度的关系
Fig.3 Relationship between k and temperature
2.2.2 颗粒度对氧化镁水化动力学的影响
在搅拌速度、温度、氧化镁浓度不变的情况下研究轻烧氧化镁颗粒度(r0)对水化率的影响,结果如图4所示。由图4可知,颗粒度控制在0.074~1.000 mm范围内,水化反应仍属于内扩散控制类型。随着氧化镁粒度的减小,水化反应速率上升。这是因为粒度的减小增大了水和氧化镁颗粒的接触面积,从而提高了反应速率。
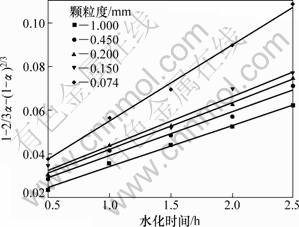
图4 颗粒度对水化动力学的影响
Fig.4 Effect of particle size on hydration kinetics
对表观反应速率和 进行线性拟合,拟合结果如图5所示。从图5可见:表观反应速率与
进行线性拟合,拟合结果如图5所示。从图5可见:表观反应速率与 成线性关系。
成线性关系。
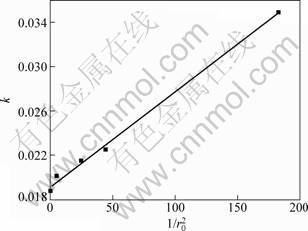
图5 颗粒度与反应速率常数的关系
Fig.5 Relationship between 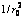 and k
and k
2.2.3 氧化镁浓度对水化动力学的影响
固定搅拌速度、反应温度及氧化镁颗粒度等条件,考察氧化镁质量浓度对水化动力学的影响,结果如图6所示。
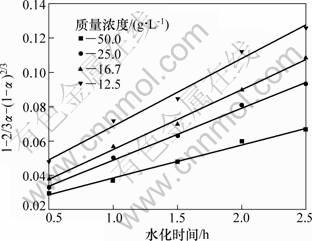
图6 氧化镁质量浓度ρ0动力学的影响
Fig.6 Effect of ρ0 on hydration kinetics
从图6可见:当氧化镁质量浓度在12.5~50 g/L之间时,轻烧氧化镁的水化反应仍然属于内扩散类型;随着氧化镁质量浓度的增加,斜率有所下降,反应速率减小。这是因为氧化镁质量浓度越大,轻烧氧化镁的含量越大,悬浮的固体颗粒物越不易分散,固液反应物之间的有效接触面积越小,在一定程度上限制了表面化学反应,导致水化速率下降。
对表观反应速率和氧化镁质量浓度进行线性拟合,拟合结果见图7。从图7可见:表观反应速率和氧化镁质量浓度存在线性关系。
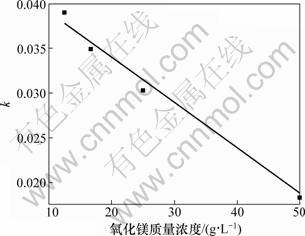
图7 氧化镁质量浓度ρ0与反应速率常数k的关系
Fig.7 Relationship between ρ0 and k
2.2.4 表观反应速率常数与温度、氧化镁浓度、颗粒度的关系
根据内扩散浸出动力学方程,在理想条件下,k与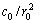 成正比[20],而根据上面的拟合结果,k与c0和
成正比[20],而根据上面的拟合结果,k与c0和 成线性关系(见图5和图7)。因此,假设它们之间存在如下关系:
成线性关系(见图5和图7)。因此,假设它们之间存在如下关系:
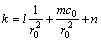 (4)
(4)
其中:l,m和n为常数。计算 和
和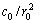 ,用MATLAB对数据进行多元线性拟合,得到
,用MATLAB对数据进行多元线性拟合,得到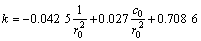 ,结合水化动力学方程,可得表观反应速率常数的经验公式为
,结合水化动力学方程,可得表观反应速率常数的经验公式为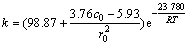 。
。
3 结论
(1) 菱镁矿的煅烧温度和煅烧时间对轻烧氧化镁的活性有较大影响,颗粒度的影响较小,最佳煅烧温度为750 ℃,保温时间为1.5 h,颗粒度为1 mm。
(2) 搅拌速度对轻烧氧化镁的水化率影响很小,根据水化动力学曲线计算出表观反应活化能为23.78 kJ/mol,两者证明氧化镁的水化反应为内扩散控制 类型。
(3) 氧化镁浓度的降低和颗粒度的减小都能提高氧化镁的水化率,通过MATLAB对数据进行拟合得到表观反应速率常数k的经验公式为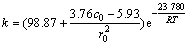 。
。
参考文献:
[1] Roco M C, Villiams R S, Alivisatos P. Nanotechnology research directions, vision for nanotechnology R&D in next decade[R]. Dordrecht: Kluwer, 2001: 43-45.
[2] Mironyuk I F, Gun’ko V M, Povazhnyak M O, et al. Magnesia formed on calcination of Mg(OH)2 prepared from natural bischofite[J]. Applied Surface Science, 2006, 252(3): 4071-4082.
[3] 胡庆福, 胡晓湘, 宋丽英. 中国专用氧化镁开发现状及发展建议[J]. 化工进展, 2005, 24(1): 30-32.
HU Qing-fu, HU Xiao-xiang, SONG Li-ying. Development status and suggestions of special magnesium oxide in China[J]. Chemical Industry and Engineering Progress, 2005, 24(1): 30-32.
[4] 郭小水, 刘家祥, 李敏, 等. 重镁水添加乙醇热解制备高纯氧化镁[J]. 有色金属, 2009, 61(1): 77-80.
GUO Xiao-shui, LIU Jia-xiang, LI Min, et al. High purity magnesia preparation from Mg(HCO3)2 with ethanol addition[J]. Nonferrous Metals, 2009, 61(1): 77-80.
[5] 谭绿贵. 龙穴山瓷石矿床地质特征及开发途径初探[J]. 矿产保护与利用, 2002(6): 27-28.
TAN Lü-gui. Geological features of longxue mountain China stone deposit and its exploitation ways[J]. Conservation and Utilization of Mineral Resources, 2002(6): 27-28.
[6] 徐徽, 苏元智, 李新海, 等. 盐湖水氯镁石制取轻质氧化镁的工艺[J]. 中国有色金属学报, 2004, 14(10): 1776-1782.
XU Hui, SU Yuan-zhi, LI Xin-hai, et al. Technology of preparation for light magnesium oxide from bischofite[J]. Transactions of Nonferrous Metals Society of China, 2004, 14(10): 1776-1782.
[7] 徐徽, 蔡勇, 石西昌, 等. 水镁石制取高纯氧化镁的研究[J]. 湖南师范大学自然科学学报, 2006, 29(1): 52-53.
XU Hui, CAI Yong, SHI Xi-chang, et al. Study on preparation of high-purity magnesia from brucite[J]. Journal of Natural Science of Hunan Normal University, 2006, 29(1): 52-53.
[8] 翟学良, 杨永杜. 活性氧化镁水化动力学研究[J]. 无机盐工业, 2004(4): 16-18.
ZHAI Xue-liang, YANG Yong-du. Study of hydrolization kinetics of activated MgO[J]. Inorganic Chemical Industry, 2004(4): 16-18.
[9] 孙永明. Statistica 6.0 研究轻烧氧化镁水化动力学[J]. 化工矿产地质, 2008, 30(4): 227-230.
SUN Yong-ming. Dynamic study of hydration of active magnesia by Statistica 6.0[J]. Geology of Chemical Mineral, 2008, 30(4): 227-230.
[10] 崔鑫, 邓敏. 氧化镁制备方法、活性与水化测定方法综述[J]. 硅酸盐通报, 2008, 27(1): 136-141.
CUI Xin, DENG Min. A review of preparation, activity and hydration of MgO[J]. Bulletin of the Chinese Ceramic Society, 2008, 27(1): 136-141.
[11] 章柯宁, 张一敏, 王昌安, 等. 从低品级菱镁矿中提取高纯氧化镁的研究[J]. 武汉科技大学学报, 2006, 29(6): 558-560.
ZHANG Ke-ning, ZHANG Yi-min, WANG Chang-an, et al. Obtaining high-grade MgO from low-grade magnesite[J]. Journal of Wuhan University of Science and Technology, 2006, 29(6): 558-560.
[12] 百丽梅, 韩跃新, 印万忠, 等. 菱镁矿制备优质活性镁技术研究[J]. 有色矿冶, 2005, 21(7): 47-48.
BAI Li-mei, HAN Yue-xin, YIN Wan-zhong, et al. Technology research on preparation of high quality active magnesia with magnesite[J]. Non-Ferrous Mining Metallurgy, 2005, 21(7): 47-48.
[13] 姜玉之, 韩跃新, 印万忠, 等. 利用菱镁矿制备氢氧化镁[J]. 东北大学学报, 2006, 27(6): 694-697.
JIANG Yu-zhi, HAN Yue-xin, YIN Wan-zhong, et al. Preparation of magnesium hydroxide from magnesite[J]. Journal of Northeastern University, 2006, 27(6): 694-697.
[14] 蒋汉瀛. 湿法冶金过程物理化学[M]. 北京: 冶金工业出版社, 1984: 89-100.
JIANG Han-ying. Physical chemistry of hydrometallurgical process[M]. BeiJing: Metallurgy Industry Press, 1984: 89-100.
[15] Bayrak B, Lacin O, Bakan F, et al. Investigation of dissolution kinetics of natural magnesite in gluconic acid solutions[J]. Chemical Engineering Journal, 2006, 117: 109-115.
[16] 钱海燕, 李素英, 邓敏, 等. 轻烧氧化镁水化动力学[J]. 化工矿物与加工, 2007 (12): 1-4.
QIAN Hai-yan, LI Su-min, DENG Min, et al. Hydration dynamic of light-burned magnesia[J]. Technology of Chemical Industrial Minerals, 2007(12): 1-4.
[17] 孙永明. 菱镁矿煅烧氧化镁制备高纯超细氢氧化镁[D]. 南京: 南京工业大学材料科学与工程学院, 2005: 29.
SUN Yong-ming. Magnesium hydroxide of high grade and extra fine obtained by hydration of light-burned magnesia calcined from magnesite[D]. Nanjing: Nanjing University of Technology. School of Material Science and Technology, 2005: 29.
[18] ZHAO Yun, ZHU Guo-cai. Thermal decomposition kinetics and mechanism of magnesium bicarbonate aqueous solution[J]. Hydrometallurgy, 2007, 89: 217-223.
[19] 李浩然, 冯雅丽, 罗小兵, 等. 湿法浸出粘土矿中钒的动力学[J]. 中南大学学报: 自然科学报, 2008, 39(6): 1181-1184.
LI Hao-ran, FENG Ya-li, LUO Xiao-bing, et al. Leaching kinetics of extraction of vanadium pentoxide from clay mineral[J]. Journal of Central South University: Science and Technology, 2008, 39(6): 1181-1184.
[20] 莫鼎成. 冶金动力学[M]. 长沙: 中南工业大学出版社, 1987: 209-215.
MO Ding-cheng. Metallurgical kinetics[M]. Changsha: Press of Central South University, 1987: 209-215.
(编辑 赵俊)
收稿日期:2011-02-06;修回日期:2011-06-01
基金项目:国家自然科学基金资助项目(20876160);国家支撑计划项目(2006BAB02A11)
通信作者:冯雅丽(1967-), 女,北京人,博士,教授,从事矿物加工方面的研究;电话:010-62311181;E-mail:ylfeng126@126.com