DOI: 10.11817/j.issn.1672-7207.2015.05.003
N, N′-二异丙氧基丙基-N″, N'''-氧二乙氧羰基硫脲在黄铜矿表面的吸附动力学和热力学特性
刘广义,张慧丽,任恒,王勇泉,肖静晶,高金亮,范佳志,钟宏
(中南大学 化学化工学院,湖南 长沙,410083)
摘要:研究一种新型结构的双硫脲表面活性剂 —— N, N′-二异丙氧基丙基-N″, N′′′-氧二乙氧羰基硫脲(DiPODECTU)在黄铜矿表面的吸附动力学和热力学。研究结果表明:黄铜矿吸附DiPODECTU较佳的pH范围为5~10,吸附量随着温度的升高而减小。等温吸附模型符合Langmuir模型,其吸附焓变△H为-19.56 kJ/mol,吸附自由能变△G为-28.70 kJ/mol(298 K),说明DiPODECTU在黄铜矿表面为单层化学吸附。吸附过程符合二级动力学方程,吸附速率常数Ks为0.144 m-2/(mol·h),平衡吸附量Qe为5.72×10-5 mol/m2,与实测值5.41×10-5 mol/m2接近。DiPODECTU主要以化学作用方式吸附在黄铜矿表面。
关键词:N, N′-二异丙氧基丙基-N″, N′′′-氧二乙氧羰基硫脲;黄铜矿;吸附性能;热力学;动力学
中图分类号:O642 文献标志码:A 文章编号:1672-7207(2015)05-1588-07
Adsorption kinetics and thermodynamics of N, N′-dipropoxypropyl-N″, N′′′-oxydiethylenedicarbonyl bis(thiourea) with chalcopyrite
LIU Guangyi, ZHANG Huili, REN Heng, WANG Yongquan, XIAO Jingjing,
GAO Jinliang, FAN Jiazhi, ZHONG Hong
(School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: A novel surfactant, N, N′-dipropoxypropyl-N″, N′′′-oxydiethylenedicarbonyl bis(thiourea) (DiPODECTU) was introduced, and its adsorption kinetics and thermodynamics with chalcopyrite were investigated. The results indicate that the recommended pHs for DiPODECTU adsorbing on chalcopyrite surface are 5-10 and the adsorption decreases with the increase of temperature. The isothermal adsorption model conforms to Langmuir model, and the adsorption enthalpy change and free energy change are -19.56 kJ/mol and -28.70 kJ/mol (298 K), respectively, which demonstrates that DiPODECTU adsorbes on chalcopyrite surface mainly through monolayer chemisorption. The adsorption process conforms to second-order kinetics equation and the adsorption rate constant is 0.144 m-2/(mol·h). The calculated equilibrium adsorption Qe (5.72×10-5 mol/m2) is close to the experimental value Qe (5.41×10-5 mol/m2). DiPODECTU adsorbs on chalcopyrite surface mainly through chemisorption.
Key words: N, N′-dipropoxypropyl-N″, N′′′-oxydiethylenedicarbonyl bis(thiourea); chalcopyrite; adsorption performance; thermodynamics; kinetics
硫脲具有生物活性[1-3]和金属螯合性能[4-6],是重要的化工原料和有机化工中间体。在农业方面[7-8],硫脲类化合物可以用作杀虫剂、除草剂、植物生长调节剂;在医药领域[9-11],硫脲类化合物具有抗病毒、抗肿瘤、抗癌和抗菌等生物和药理活性;在冶金和分析方面[12-16],硫脲类化合物常用于贵金属的分离和富集,也可用于电镀和化学镀;在矿物加工工程领域[17-20],常采用硫脲类捕收剂来强化贵金属的浮选回收。分子中含1个烷氧羰基和1个硫脲基的单烷氧羰基硫脲表面活性剂是铜矿物和贵金属的特效捕收剂,它们在弱碱性或中性pH矿浆中表现出对黄铜矿强的捕收能力[7-20],但分子中含2个烷氧羰基和2个硫脲基的二酯基双硫脲用作铜矿物捕收剂的报道较少。二酯基双硫脲分子中配位原子和基团的增加会提高其与金属离子的络合能力,强化其在矿物表面的吸附,因此有望成为一类新型的螯合分子和螯合浮选表面活性剂,用来提高金属矿山中有价金属元素的回收[21]。在此,本文作者研究一种新型结构的双硫脲表面活性剂 —— N, N′-二异丙氧基丙基-N″, N′′′-氧二乙氧羰基硫脲(DiPODECTU)在黄铜矿表面的吸附行为及其吸附热力学与动力学特性,并采用红外光谱探讨DiPODECTU与铜/亚铜离子的作用及其在黄铜矿表面的吸附机理。
1 实验
1.1 试剂与仪器
实验试剂包括无水乙醇(分析纯)、N, N′-二异丙氧基丙基-N″, N′′′-氧二乙氧羰基硫脲(DiPODECTU)(自制,纯度98%)、蒸馏水和黄铜矿(取自德兴铜矿,为手捡分选样品)。实验仪器为UV-2100 型紫外可见分光光度计、pHS-3C型pH计、Nicolet FTIR-740 型傅里叶变换红外光谱仪和SHA-C型水浴恒温振荡器。
1.2 矿样的制备
黄铜矿经过手碎、手选和玛瑙研钵研磨,并用孔径为75 μm和38 μm的筛子筛分,取粒径为38~75 μm的矿样用于吸附试验,Cu,Fe和S元素质量分数分别为34.56%,30.52%和34.92%,测得比表面积为0.08 m2/g。实验前,用超声波洗涤矿样3~4次,每次洗涤2 min后抽滤,自然晾干。
1.3 DiPODECTU溶液的线性关系
配置一定浓度梯度的DiPODECTU溶液(pH为8.5左右),在特征波长258 nm处,用紫外分光光度计测定其吸光度(A),然后以吸光度为纵坐标,浓度(X)为横坐标,得线性回归方程:A=0.040 6+0.250 97X,R2=0.999 51。
1.4 吸附实验
量取50 mL一定初始浓度的DiPODECTU溶液于100 mL锥形瓶中,再加入0.5 g黄铜矿,根据需要调节溶液pH或温度,置于恒温水浴振荡器中,转速为200 r/min,25 ℃下震荡一段时间,然后离心取上层清液测定其吸光度。
根据所得数据按照式(1)计算出任意时刻吸附量Qt。
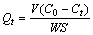 (1)
(1)
式中:C0为DiPODECTU溶液初始浓度,mol/L;Ct为任意时刻DiPODECTU溶液浓度,mol/L;V为溶液体积,L;W为黄铜矿的加入量,g;Qt为任意时刻黄铜矿的吸附量,mol/m2;S为黄铜矿的比表面积,m2/g。
1.5 红外光谱
药剂与金属离子或矿物作用前后的红外光谱在Nicolet FTIR-740 型傅里叶变换红外光谱仪上采用溴化钾压片法进行红外检测,波数范围为4 000~400 cm-1。将药剂与金属离子或矿物作用后的固体经真空干燥后进行红外检测。纯矿物磨细后直接用于红外检测。
2 结果与讨论
2.1 时间对吸附量的影响
在DiPODECTU初始浓度为5×10-5 mol/L,温度为298 K时,黄铜矿对DiPODECTU的吸附量随时间变化的关系如图1所示,由图1可知:黄铜矿对DiPODECTU的吸附量随着吸附时间的延长而增大,在吸附过程开始的0~10 h内,黄铜矿对DiPODECTU的吸附速率最大,吸附量增长最快。吸附10 h之后,随着时间的延长,吸附量增加变缓,12 h时吸附量基本达到饱和,再延长吸附时间,吸附量没有明显的提高。
2.2 pH对吸附量的影响
在吸附时间为12 h,温度为298 K时,溶液pH对黄铜矿吸附DiPODECTU的影响见图2。图2表明:随着pH的增加,黄铜矿对DiPODECTU的吸附量先增加后减少,pH约为8.5时达到最大。当pH为5~10时,DiPODECTU在黄铜矿表面的吸附量较大,强酸性或强碱性条件下都不利于其在黄铜矿表面的吸附。
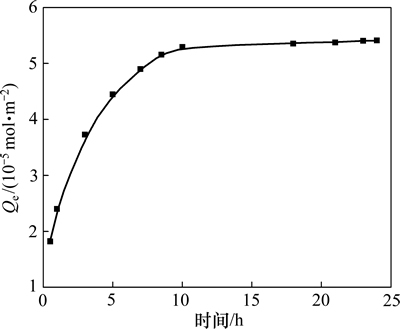
图1 黄铜矿对DiPODECTU的吸附量随时间变化的关系曲线
Fig. 1 Adsorption of DiPODECTU on chalcopyrite surface as a function of time
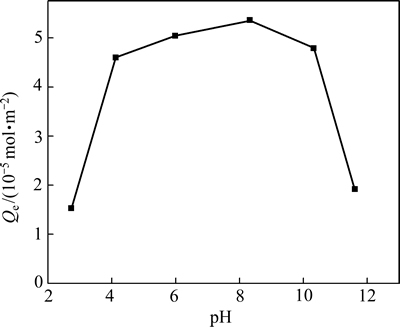
图2 黄铜矿对DiPODECTU的吸附量与溶液pH的关系
Fig. 2 Adsorption of DiPODECTU on chalcopyrite surface as a function of pH
2.3 等温吸附模型
在吸附时间为12 h,pH 8.5左右,3种不同温度(298,318和328 K)下黄铜矿对DiPODECTU的平衡吸附量(Qe)与DiPODECTU的平衡浓度(Ce)的关系见图3。
由图3可知:在同一温度下,随着DiPODECTU平衡浓度的增加,黄铜矿对DiPODECTU的平衡吸附量也增加;在相同的平衡浓度条件下,黄铜矿对DiPODECTU的平衡吸附量随温度的升高反而降低,表明该吸附过程为放热过程。
分别采用Langmuir(式(2))、Freundlich(式(3))等温吸附方程对图3中实验数据进行线性拟合,并对两者进行了比较,结果见图4和表1以及图5和表2。
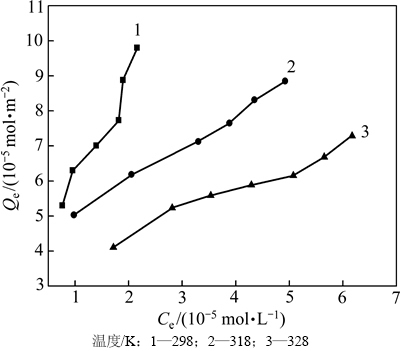
图3 黄铜矿对DiPODECTU的吸附等温线
Fig. 3 Adsorption isotherm of DiPODECTU on chalcopyrite surface
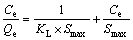 (2)
(2)
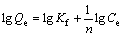 (3)
(3)
式中:KL为Langmuir常数;Smax为单位体积最大吸附容量,mol/m2;Kf和n为Freundlich参数。
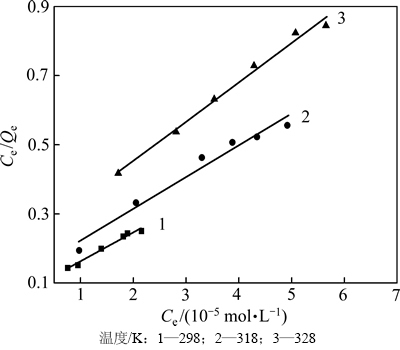
图4 Langmuir拟合曲线
Fig. 4 Langmuir fitting curves
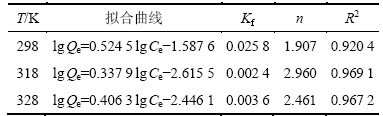
图4和表1以及图5和表2的拟合结果表明:在实验范围内,黄铜矿对DiPODECTU的吸附可认为既符合Langmuir也符合Freundlich等温吸附方程(R2>0.90),但Langmuir等温吸附方程拟合的线性相关性更佳(R2>0.95),说明黄铜矿表面上的吸附形式以单层化学吸附为主。
表1 Langmuir等温方程拟合结果
Table 1 Fitting results of Langmuir Isotherm Equation

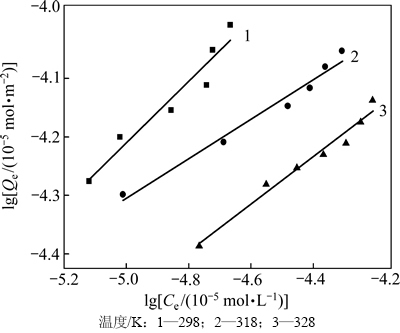
图5 Freundlich拟合曲线
Fig. 5 Freundlich fitting curve
表2 Freundlich等温方程拟合结果
Table 2 Fitting results of Freundlich Isotherm Equation
2.4 吸附热力学
吸附熵变(△S)、吸附焓变(△H)和吸附自由能变(△G)是测定吸附过程的自发性和热量变化的重要热力学参数,能够反映出温度对DiPODECTU在黄铜矿表面吸附行为的影响。上述热力学参数可以通过式(4)和式(5)进行计算[22]。
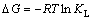 (4)
(4)
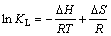 (5)
(5)
其中:R为热力学气体常数,8.314 J/(mol·K);T为热力学温度,K;KL为Langmuir常数。
△H和△S可通过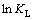 对1/T作图所得斜率和截距求得,结果见表3和图6。从表3和图6可知:在298,318和328 K下,△G均为负值,表明这3种温度下的吸附过程均为自发过程。△H为负值表明,DiPODECTU在黄铜矿表面的吸附过程为放热过程,高温不利于吸附,这与吸附等温线的实验结果一致。
对1/T作图所得斜率和截距求得,结果见表3和图6。从表3和图6可知:在298,318和328 K下,△G均为负值,表明这3种温度下的吸附过程均为自发过程。△H为负值表明,DiPODECTU在黄铜矿表面的吸附过程为放热过程,高温不利于吸附,这与吸附等温线的实验结果一致。
表3 DiPODECTU在黄铜矿表面吸附的热力学参数
Table 3 Thermodynamics parameters of DiPODECTU adsorbing on chalcopyrite surface

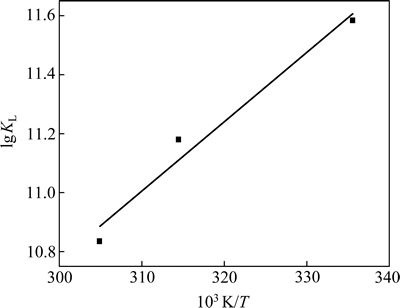
图6 ln KL与1/T关系图
Fig. 6 Relationship between ln KL and 1/T
2.5 吸附动力学
采用二级反应动力学方程[23](式(6))对DiPODECTU在黄铜矿表面的吸附过程(实验数据见图1)拟合,其t/Qt-t关系见图7。
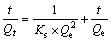 (6)
(6)
式中:Ks为吸附速率常数,m-2/(mol·h)。
由图7可知:DiPODECTU在黄铜矿表面吸附的t/Qt-t有很好的线性关系(R2>0.999),表明吸附过程符合二级动力学方程。通过计算可得到吸附速率常数Ks为0.144 m-2/(mol·h),平衡吸附量Qe为5.72×10-5 mol/m2,与实测值5.41×10-5 mol/m2十分接近。
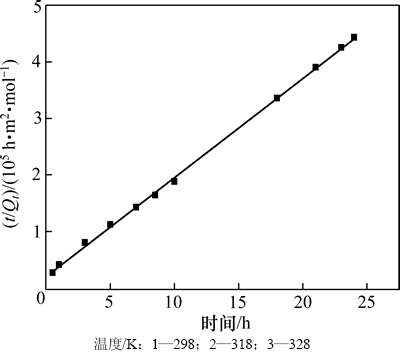
图7 DiPODECTU在黄铜矿表面上的吸附动力学
Fig. 7 Adsorption dynamics of DiPODECTU on chalcopyrite surface
2.6 与金属离子的作用
DiPODECTU与Cu2+/Cu+作用产物(DiPODECTU+ Cu2+/DiPODECTU+Cu+)的红外光谱如图8所示。
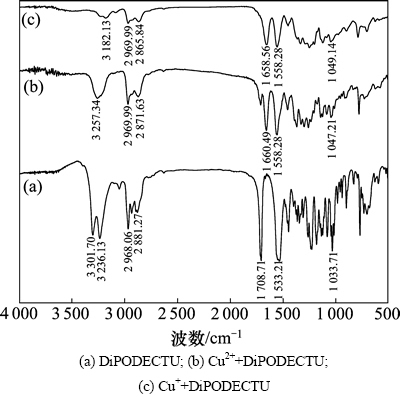
图8 DiPODECTU与Cu2+/Cu+作用产物的红外光谱
Fig. 8 IR spectra of DiPODECTU binding with Cu2+/Cu+
由图8可知:与DiPODECTU相比,DiPODECTU+ Cu2+和DiPODECTU+Cu+的红外光谱中特征峰的强度或者位移都发生了很明显的变化:在3 302~3 182 cm-1附近,DiPODECTU+Cu2+和DiPODECTU+Cu+中的 N—H伸缩振动峰强度明显减弱,有些甚至消失,可能是由于原本DiPODECTU里的2个N—H中的1个与Cu2+/Cu+反应形成了新键导致N—H键分裂所致;在1 660 cm-1和1 659 cm-1处,C=O伸缩振动变化非常明显,DiPODECTU+Cu2+中由原来1 709 cm-1处向低频方向移动了49 cm-1,DiPODECTU+Cu+中由原来1 709 cm-1处向低频方向移动了50 cm-1,可能是由于Cu2+的加入使—C(=S)—N—C(=O)—中大π键电子增加离域性增大,导致振动频率下降,这与DEOECTU与铜离子反应前后红外特征峰位移的情况相似[24]。DiPODECTU在1 533 cm-1处的—C(=S)—NH—复合振动峰在DiPODECTU+Cu2+和DiPODECTU+Cu+中都移向高频1 558 cm-1处,可能是由于C—N双键成分增加导致其键能增大、键长缩短的原因[21, 25-26]。1 034 cm-1(DiPODECTU)处的C=S伸缩振动移向1 047 cm-1(DiPODECTU+Cu2+)或1 049 cm-1(DiPODECTU+ Cu+)附近,可能C=S中的S原子参与和铜的成键,由于C—S伸缩振动稍微向高频移动,暗示新生成的Cu—S键中可能同时存在正配键和反馈配键。因此,初步推断当Cu2+/Cu+与DiPODECTU反应时,C(=S)—NH—C(=O)基团重排形成HS—C=N—C(=O),Cu2+/Cu+与S生成Cu—S键,同时释放出H+。通过以上分析,可以确定DiPODECTU与Cu2+/Cu+发生了化学反应,生成了新的络合物,并有氢离子释放。
2.7 与矿物作用的漫反射红外光谱
黄铜矿与DiPODECTU作用前后的漫反射红外光谱见图9。
由图9可知:与黄铜矿红外光谱曲线(图9(a))相比,黄铜矿吸附DiPODECTU后的红外光谱曲线(图9(b))在2 967和2 866 cm-1处出现C—H伸缩振动,在1 558 cm-1处出现了—C(=S)—N—复合伸缩振动特征吸收峰,这与DiPODECTU与Cu2+/Cu+作用产物中的1 558 cm-1处的—C(=S)—N—复合伸缩振动相吻合;在1 651 cm-1处出现的吸收峰,与DiPODECTU和Cu2+/Cu+作用产物中1 660 cm-1/1 658 cm-1处的吸收峰相吻合;在1 715 cm-1处出现的弱吸收峰,与DiPODECTU中C=O伸缩振动在1 709 cm-1出现的吸收峰相一致。这充分说明,DiPODECTU通过与黄铜矿表面的Cu2+/Cu+发生化学反应而吸附在其表面。并且,图9(b)中,与在1 651 cm-1处(DiPODECTU和Cu2+/Cu+的作用产物)的吸收峰相比,在1 715 cm-1处(DiPODECTU分子) C=O伸缩振动吸收峰相对较弱,这表明大部分DiPODECTU以化学方式吸附在黄铜矿表面,但DiPODECTU含双—C(=S)—N—C(=O)—官能团,可能小部分DiPODECTU分子中只有1个 —C(=S)—N—C(=O)—化学吸附在黄铜矿表面,而另一个C(=S)—N—C(=O)—却保持流离,因此黄铜矿吸附DiPODECTU后还在1 715 cm-1处(DiPODECTU分子)出现了C=O伸缩振动吸收峰。红外光谱进一步证实DiPODECTU以化学方式吸附在黄铜矿表面,这与吸附热力学的结论一致。
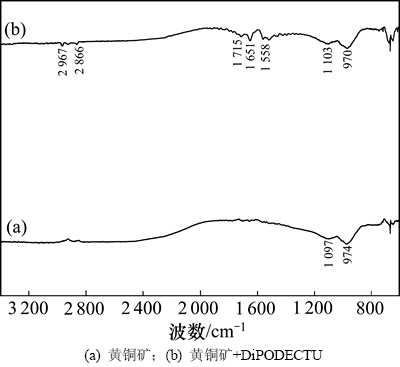
图9 黄铜矿吸附DiPODECTU前后的红外光谱图
Fig. 9 Diffuse reflectance infrared spectra of chalcopyrite before and after adsorption of DiPODECTU
3 结论
1) 黄铜矿吸附DiPODECTU较佳的pH范围为5~10,吸附量随着温度的升高而减小。等温吸附模型符合Langmiur模型,其吸附焓变△H为-19.56 kJ/mol,吸附自由能变△G为-28.70 kJ/mol(298 K),说明DiPODECTU在黄铜矿表面为单层化学吸附。吸附过程符合二级动力学方程,吸附速率常数Ks为0.144 m-2/(mol·h),平衡吸附量Qe为5.72×10-5 mol/m2,与实测值5.41×10-5 mol/m2接近。
2) DiPODECTU与Cu2+/Cu+反应生成了新化合物,且DiPODECTU主要以化学作用方式吸附在黄铜矿表面。
参考文献:
[1] LIU Guangyi, QIU Zhaohui, WANG Jingyi, et al. Study of N-isopropoxyprophyl-N′-ethoxycarbonyl thiourea adsorption on chalcopyrite using in situ SECM, ToF-SIMS and XPS[J]. Journal of Colloid Interface Science, 2015, 437: 42-49.
[2] Rodger A, Patel K K, Sanders K J, et al. Anti-tumour platinum acylthiourea complexes and their interactions with DNA[J]. Journal of the Chemical Society, Dalton Transactions, 2002, 19(19): 3656-3663.
[3] HU Jinghan, WANG Liangcheng, LIU Hong, et al. Biological activities studies and phase transfer catalysts promoting the one-pot synthesis of N-aryl-N′-(4-ethyloxy benzoyl)thiourea derivatives[J]. Phosphorus, Sulfur, and Silicon and the Related Elements, 2006, 181(12): 2691-2698.
[4] Koch K R. New chemistry with old ligands: N-alkyl- and N, N-dialkyl-N′-acyl(aroyl) thioureas in co-ordination, analytical and process chemistry of the platinum group metals[J]. Coordination Chemistry Reviews, 2001, 216/217: 473-488.
[5] Habtu M M, Bourne S A, Koch K R, et al. Competitive bulk liquid membrane transport and solvent extraction of some transition and post-transition metal ions using acylthiourea ligands as ionophores[J]. New Journal of Chemistry, 2006, 30: 1155-1162.
[6] Guillon E, Mohamadou A, Digchamps-Olivier I, et al. Synthesis and characterization of copper, nickel and cobalt complexes with N-disubstituted, N'-ethoxycarbonyl thioureas[J]. Polyhedron, 1996, 15(5/6): 947-952.
[7] 匡宇明, 李中华. N,N-二(2-氯乙基)-磷酰基双硫脲的合成及除草活性研究[J]. 化学通报, 2004, 67(6): 449-451.
KUANG Yuming, LI Zhonghua. Studies on the synthesis and herbicidal activities of N,N-di(2-chloroethenyl)-phosphoryl- bithioureas[J]. Chemistry Online, 2004, 67(6): 449-451.
[8] 李淑贤, 王焱钢. N′-5-四唑基-N-芳甲酰基硫脲的合成及其生物活性研究(Ⅰ)[J]. 有机化学, 2003, 23(11): 1311-1313.
LI Shuxian, WANG Yangang. Synthesis and Biological activity of N′-5-Tetrazolyl-N-aryl formyl thiourea(Ⅰ)[J]. Chinese Journal of Organic Chemistry, 2003, 23(11): 1311-1313.
[9] 胡惟孝, 孙楠, 杨忠愚. 缩胺硫脲化合物的合成及其抗癌活性的研究[J]. 高等学校化学学报, 2001, 22(12): 2014-2017.
HU Weixiao, SUN Nan, YANG Zhongyu. Studies on synthesis and anticancer activity of Thiosemicarbazones[J]. Chemical Journal of Chinese Universities, 2001, 22(12): 2014-2017.
[10] Song B A, Jin L H, Yang S, et al. Synthesis and antiviral activities of Chiral Thiourea Derivatives[J]. Chinese Journal of Chemistry, 2009, 27(3): 593-601
[11] Maddani M R, Prabhu K R. A concise synthesis of substituted thiourea derivatives in aqueous medium[J]. Journal of Organic Chemistry, 2010, 75(7): 2327-2332.
[12] 王云燕, 柴立元. 硫脲在碱性介质中的电化学行为[J]. 中国有色金属学报, 2008, 18(4): 733-737.
WANG Yunyan, CHAI Liyuan. Electrochemical behaviors of thiourea in alkaline medium[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(4): 733-737.
[13] Shen C B, Han D Y, Ding Z M. The inhibition effect of thiourea on bulk nanocrystallized ingot iron in acidic sulfate solution[J]. Materials Chemistry and Physics, 2008, 109: 417-421.
[14] ZHON Limin, WANG Yiping, LIU Zhirong, et al. Characteristics of equilibrium, kinetics studies for adsorption of Hg(Ⅱ), Cu(Ⅱ), and Ni(Ⅱ) ions by thiourea-modified magnetic chitosan microspheres[J]. Journal of Hazardous Materials, 2009, 161: 995-1002.
[15] Ramesh A, Hasegawa H, Sugimoto W, et al. Adsorption of gold(Ⅲ), platinum(Ⅳ) and palladium(Ⅱ) onto glycine modified crosslinked chitosan resin[J]. Bioresource Technology, 2008, 99: 3801-3809.
[16] Birinci E, Gulfen M, Aydin A O. Separation and recovery of palladium(Ⅱ) from base metal ions by melamine-formaldehyde- thiourea (MFT) chelating resin[J]. Hydrometallurgy, 2009, 95: 15-21.
[17] Fairthorne G, Fornasiero D, Ralston J. Interaction of thionocarbamate and thiourea collectors with sulfide minerals: A flotation and adsorption study[J]. International Journal of Mineral Processing, 1997, 50: 227-242.
[18] LIU Guangyi, ZHONG Hong, XIA Liuyin, et al. Improving copper flotation recovery from a refractory copper porphyry ore by using ethoxycarbonyl thiourea as a collector[J]. Minerals Engineering, 2011, 24: 817-824.
[19] LIU Guangyi, ZHONG Hong, XIA Liuyin, et al. Effect of N-substituents on performance of thiourea collectors by density functional theory calculations[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(4): 695-701.
[20] 刘广义, 钟宏, 戴塔根, 等. 中碱度条件下乙氧羰基硫脲浮选分离铜硫[J]. 中国有色金属学报, 2009, 19(2): 389-396.
LIU Guangyi, ZHONG Hong, DAI Tagen, et al. Flotation separation of Cu/Fe sulfide minerals by ethoxycarbonyl thiourea under middle alkaline conditions[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(2): 389-396.
[21] 刘广义, 任恒, 詹金华, 等. 3, 3′-二乙基-1, 1′-一缩二乙二醇二羰基双硫脲的合成、表征与性能[J]. 中国有色金属学报, 2013, 23(1): 290-296.
LIU Guangyi, REN Heng, ZHAN Jinhua, et al. Synthesis, characterization and properties of 3,3′-diethyl-1,1′- oxydiethylenedicarbonyl bis(thiourea)[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(1): 290-296.
[22] Al-Ghouti M, Khraisheh M A M, Ahmad M N M, et al. Thermodynamic behavior and the effect of temperature on the removal of dyes form aqueous solution using modified diatomite: A kinetic study[J]. Journal of Colloid Interface Science, 2005, 287(1): 6-13
[23] Ho Y, Mckay G. Pseudo-second order model for sorption processes[J]. Process Biochemistry, 1999, 34(5): 451-465.
[24] LIU Guangyi, REN Heng, ZHAN Jinhua, et al. Synthesis, characterization and properties of 3,3′-diethyl-1,1′- oxydiethylenedicarbonyl bis(thiourea)[J]. Research on Chemical Intermediates, 2014, 10(5): 2025-2038.
[25] 刘广义. 硫化铜矿石的综合利用及新型捕收剂研究[D]. 长沙: 中南大学化学化工学院, 2004: 15-38.
LIU Guangyi. The research on comprehensive utilization for copper sulfide ores with new collectors[D]. Changsha: Central South University. School of Chemistry and Chemical Engineering, 2004: 15-38.
[26] Fairthorne G. The interaction of thionocarbamate and thiourea collectors with sulfide mineral surfaces[D]. Australia: University of South Australia. Ian Wark Research Institute, 1996: 20-45.
(编辑 赵俊)
收稿日期:2015-03-08;修回日期:2015-05-28
基金项目(Foundation item):国家自然科学基金资助项目(51074183);国家高技术研究发展计划(863计划)项目(2013AA064101);全国优秀博士学位论文作者专项资金资助项目(2007B52);教育部新世纪优秀人才支持计划项目(NCEP-08-0568) (Project(51074183) supported by the National Natural Science Foundation of China; Project (2013AA064101) supported by the National High Technology Research and Development Program of China (863 Program); Project(2007B52) supported by the Foundation for the Author of National Excellent Doctoral Dissertation of China; Project (NCEP-08-0568) supported by the Program for New Century Excellent Talents in Chinese University)
通信作者:刘广义,博士,教授,从事有色金属资源综合回收与高效利用研究;E-mail: guangyi.liu@163.com