
Preparation of copper arsenite and its application in
purification of copper electrolyte
XIAO Fa-xin(肖发新)1, ZHENG Ya-jie(郑雅杰)1, WANG Yong(王 勇)1, 2,
JIAN Hong-sheng(简洪生)1, 2, LI Chun-hua(李春华)1, 2, XU Wei(许 卫)1, 2, MA Yu-tian(马玉天)1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. Daye Nonferrous Metal Limited Co. Ltd., Huangshi 435005, China
Received 21 March 2007; accepted 12 July 2007
Abstract: The preparation of copper arsenite with arsenic trioxide was presented and its application in the purification of copper electrolyte was proposed. The variables of n(OH-)/n(As), n(Cu)/n(As), NaOH concentration, reaction temperature and pH value have some effects on the yield of copper arsenite. The optimum conditions of preparing copper arsenite are that the molar ratio of alkali to arsenic is 2:1, NaOH concentration is 1 mol/L, the molar ratio of copper to arsenic is 2:1, pH value is 6.0 and reaction temperature is 20 ℃. The yield of copper arsenite is as high as 98.65% under optimum conditions and the molar ratio of Cu to As in the product is about 5:4. The results of the purification experiments show that the removal rate of antimony and bismuth is 53.85% and 53.33% respectively after 20 g/L copper arsenite is added. The purification of copper electrolyte with copper arsenite has the advantages of simple technique, good purification performance and low cost.
Key words: arsenic trioxide; copper arsenite; purification; copper electrolyte
1 Introduction
Arsenic broadly exists in the mines of lead, copper, silver, antimony, iron and other metals[1-2]. On one hand, there are about several hundred thousands tons of arsenic cycling in the steps of mining, beneficiation and smelting each year during the producing process of nonferrous metals in China[3-5]; on the other hand, arsenic and its compounds are toxic, the disposal technique is complicated and the demand of arsenic is limited[6-7]. Therefore, how to effectively dispose and use arsenic compounds is a hot potato for many smelting plants[8-10].
In 18th century, the Sweden chemist Scheele firstly discovered copper arsenite and took it as green paint[11]. In the middle period of 19th century, a kind of pesticide mainly composed of copper arsenite was firstly registered in America[12]. Recently, it was found by authors that copper arsenite can be used to purify copper electrolyte. The concentration of antimony and bismuth in copper electrolyte greatly decreased after copper arsenite was added. However, there was less report about the preparation technique of copper arsenite in the world. The preparation method of copper arsenite with arsenic trioxide was presented and its application in the purification of copper electrolyte was firstly proposed in this work.
2 Experimental
All the experiments were conducted in a beaker of 0.5 L, which was stirred mechanically (200-400 r/min) in a thermostatic water bath. Reagent grade As2O3, NaOH, CuSO4·5H2O and concentrated H2SO4 were used in the preparation of copper arsenite.
2.1 Preparation of copper arsenite
Copper arsenite was prepared by dissolving copper sulphate in arsenious solution that was obtained by dissolving As2O3 in NaOH solution. Concentrated H2SO4 was used to adjust pH value of the solution. The quantitative contents of Cu and As of product were analyzed by chemical analysis. The structure of product was detected by X-ray diffractometer (D/max-rA, Rigaku Corporation of Japan).
2.2 Purification of copper electrolyte
The purification experiment was carried out by dissolving copper arsenite in the electrolyte under stirring for several hours. The purified electrolyte was then filtered to remove the precipitate. The content of antimony and bismuth in the electrolyte was determined by ICP emission spectroscopy( IRIS Intrepid II XSP, Thermo Elemental Corporation, America) and the content of Cu, H2SO4 and As by chemical analysis.
3 Results and discussion
3.1 Impact of n(OH-)/n(As) on yield of copper arsenite
The impact of n(OH-)/n(As) (the molar ratio of hydroxyl to arsenic) on the yield of copper arsenite is presented in Fig.1. The technical conditions are n(Cu)/n(As)=3?2, NaOH solution concentration 3 mol/L, pH value 6.0 and reaction temperature 20 ℃.
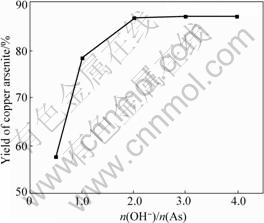
Fig.1 Impact of n(OH-)/n(As) on yield of copper arsenite
A kind of white precipitate is found on the surface of solution during the preparation experiments. What’s more, some precipitate is formed in the bottom of the beaker when n(OH-)/n(As) is less than or equal to 1?1. The XRD pattern of the precipitate is shown in Fig.2.
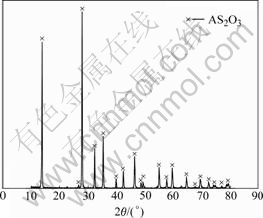
Fig.2 XRD pattern of precipitate when n(OH-)/n(As)≤1
It can be seen from Fig.1 that the yield of copper arsenite increases with the increase of n(OH-)/n(As) up to 2?1 and then remains constant with the further increase. Fig.2 shows that the white precipitate is As2O3. The reaction occurs as follows when As2O3 is dissolved in NaOH solution:
As2O3+2NaOH→2NaAsO2+H2O (1)
Obviously, As2O3 can not dissolve completely when n(OH-)/n(As) is less than 1?1. The higher the n(OH-)/n(As) is, the higher the 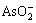 content and the more the mass of copper arsenite, therefore the higher the yield. When n(OH-)/n(As) is more than 2?1, As2O3 is completely dissolved in NaOH solution, then the yield of copper arsenite remains constant. Therefore, the suitable n(OH-)/n(As) is 2?1 in the preparation of copper arsenite.
content and the more the mass of copper arsenite, therefore the higher the yield. When n(OH-)/n(As) is more than 2?1, As2O3 is completely dissolved in NaOH solution, then the yield of copper arsenite remains constant. Therefore, the suitable n(OH-)/n(As) is 2?1 in the preparation of copper arsenite.
3.2 Impact of NaOH concentration on yield of copper arsenite
The impact of NaOH concentration on the yield of copper arsenite is shown in Fig.3. The conditions are n(OH-)/n(As)=2?1 and other conditions fixed.
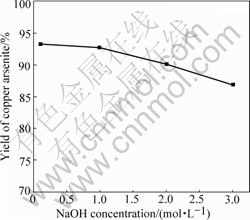
Fig.3 Impact of NaOH concentration on yield of copper arsenite
It is obvious from Fig.3 that the yield of copper arsenite decreases with the increase of NaOH concentration. A kind of white precipitate is found on the surface of solution when adjusting pH value by H2SO4 if the NaOH concentration is too high. This is because that the solubility of As(Ⅲ) decreases and some As2O3 form in the solution when pH value decreases. The reaction occurs as follows when concentrated H2SO4 is added:
2H++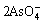 →As2O3+H2O (2)
→As2O3+H2O (2)
The volume of the solution increases sharply and the operation is inconvenient when NaOH concentration is too low although the yield is high. Therefore, the suitable NaOH concentration is 1 mol/L in the prepara- tion of copper arsenite.
3.3 Impact of n(Cu)/n(As) on yield of copper arsenite
The impact of n(Cu)/n(As) on the yield of copper arsenite is shown in Fig.4. The conditions are that NaOH concentration was 1 mol/L and other conditions were fixed.
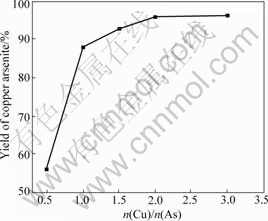
Fig.4 Impact of n(Cu)/n(As) on yield of copper arsenite
From Fig.4, it can be seen that the yield of copper arsenite increases with the increase of n(Cu)/n(As) up to 2?1 and then remains nearly constant with further increase. The suitable n(Cu)/n(As) is 2?1 in the preparation of copper arsenite.
3.4 Impact of pH value on yield of copper arsenite
The impact of pH value on the yield of copper arsenite is shown in Fig.5. The conditions are n(Cu)/ n(As) =2?1 and other conditions fixed.
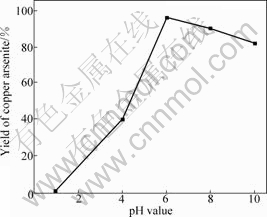
Fig.5 Impact of pH value on yield of copper arsenite
It can be seen from Fig.5 that the yield of copper arsenite increases firstly and then decreases with the increase of pH value. It is found that a kind of white product is found on the button of solution when pH value is 1.0, a kind of yellow-green product when pH value is 4.0, a kind of green product when pH value is 6.0 and a kind of thin green product when pH value is 10.0. The XRD results of these products are shown in Figs.6-9 respectively.
Fig.6 XRD pattern of precipitate at pH=1
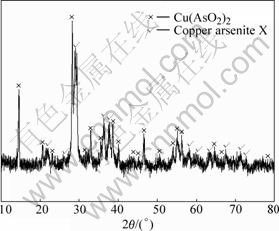
Fig.7 XRD pattern of product at pH=4
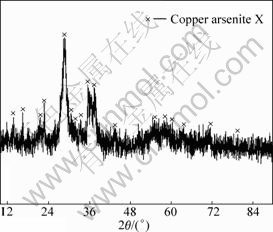
Fig.8 XRD pattern of product at pH=6
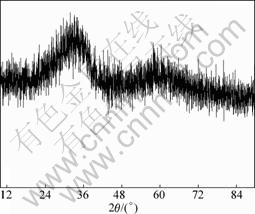
Fig.9 XRD pattern of product at pH=10
It can be seen from Figs.6-9 that the white precipitate is As2O3, the yellow-green product is (Cu(AsO2)2) and copper arsenite X, the green copper arsenite is copper arsenite X and the thin green product obtained at pH=10.0 is amorphous. The structure of copper arsenite X remains to be studied further as there is no corresponding JCPDS.
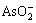 forms in the solution when As2O3 is dissolved in NaOH solution. Some
forms in the solution when As2O3 is dissolved in NaOH solution. Some 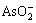 ions in solution react with H+ to form As2O3 when concentrated H2SO4 is added to adjust pH value to 1.0, the rest exist in the form of
ions in solution react with H+ to form As2O3 when concentrated H2SO4 is added to adjust pH value to 1.0, the rest exist in the form of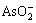 state, therefore the yield of copper arsenite is 0. The reactions occur as follows:
state, therefore the yield of copper arsenite is 0. The reactions occur as follows:
As2O3+2OH-→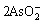 +H2O (3)
+H2O (3)
2H++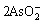 →As2O3+H2O (4)
→As2O3+H2O (4)
When pH value is 4.0, some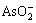 ions react with Cu2+ to form copper arsenite X, some react with Cu2+ to form Cu(AsO2)2, others remain in the form of
ions react with Cu2+ to form copper arsenite X, some react with Cu2+ to form Cu(AsO2)2, others remain in the form of  therefore the yield of copper arsenite is low. The reactions occur as follows:
therefore the yield of copper arsenite is low. The reactions occur as follows:
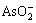 +Cu2+→Copper arsenite X (5)
+Cu2+→Copper arsenite X (5)
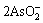 +Cu2+→Cu(AsO2)2↓ (6)
+Cu2+→Cu(AsO2)2↓ (6)
Almost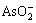 ions react with Cu2+ to form copper arsenite X in the solution when pH value is 6.0, therefore the yield is high. A kind of amorphous copper arsenite is prepared under pH value of 10 and its yield is not high. Therefore the suitable pH value is 6.0 in the preparation of copper arsenite.
ions react with Cu2+ to form copper arsenite X in the solution when pH value is 6.0, therefore the yield is high. A kind of amorphous copper arsenite is prepared under pH value of 10 and its yield is not high. Therefore the suitable pH value is 6.0 in the preparation of copper arsenite.
3.5 Impact of reaction temperature on yield of copper arsenite
The impact of reaction temperature on the yield of copper arsenite is shown in Fig.10. The conditions are pH value 6.0 and other conditions fixed.
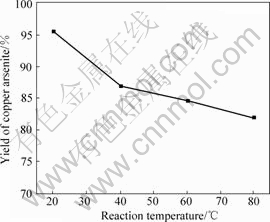
Fig.10 Impact of reaction temperature on yield of copper arsenite
It is obvious from Fig.10 that the yield of copper arsenite decreases with the increase of reaction temperature. Obviously, the solubility of copper arsenite increases with the increase of reaction temperature, which results in the decrease of yield. Therefore the suitable reaction temperature is 20℃ in the preparation of copper arsenite.
The results above show that the optimum conditions of preparing copper arsenite are that n(OH-)/n(As) is 2?1, NaOH solution concentration is 1 mol/L, n(Cu)/n(As) is 2?1, pH value is 6.0 and reaction temperature is 20 ℃.
Under the optimum conditions, 80 g green copper arsenite was prepared with 40 g As2O3. The contents of Cu and As are 39.18% and 37.00% respectively in the product. Therefore the yield of copper arsenite is 98.65% and the molar ratio of Cu to As is nearly 5?4.
3.6 Results of copper electrolyte purification
The impact of copper arsenite concentration on removal rate of Sb and Bi is shown in Fig.11.
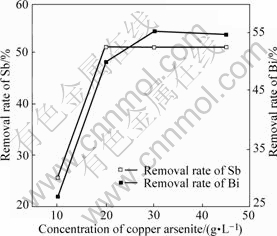
Fig.11 Impact of concentration of copper arsenite on removal rate of Sb and Bi
From Fig.11, it can be seen that the removal of Sb and Bi increases with the increase of copper arsenite concentration up to 20 g/L and then remains constant with further increase, which indicates that copper arsenite can be used to purify copper electrolyte and the appropriate addition of copper arsenite is 20 g/L. The electrolyte components before and after purification are listed in Table 1, which was obtained by adding 20 g copper arsenite in 1 L copper electrolyte.
Table 1 Electrolyte components before and after purification (g/L)
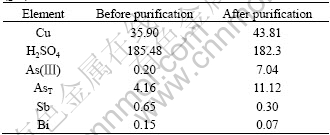
It can be seen from Table 1 that the removal rates of Sb and Bi in electrolyte are as high as 53.85% and 53.33% respectively when the concentrations of As(Ⅲ) and AsT are up to 7.04 and 11.12 g/L. The total content of As, Sb and Bi of precipitate is 56.94% by chemical analysis, which shows that some kind of precipitate mainly composed of As, Sb and Bi forms in electrolyte and therefore Sb and Bi are removed from electrolyte. The purification results of synthesized electrolyte show that three precipitates form in electrolyte by As, Sb, Bi. Precipitate A is formed by As(Ⅲ), Sb(Ⅲ), Sb(Ⅴ), precipitate B by As(Ⅴ), Sb(Ⅴ), As(Ⅲ) and precipitate C by As(Ⅴ), Sb(Ⅴ), Bi(Ⅲ). The XRD results of these precipitate are shown in Figs.12-14, respectively.
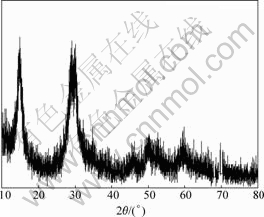
Fig.12 XRD pattern of precipitate A
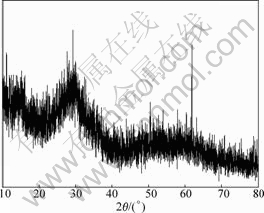
Fig.13 XRD pattern of precipitate B
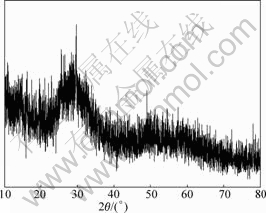
Fig.14 XRD pattern of precipitate C
It can be seen from Figs.12-14 that precipitate A is a kind of crystal compound, while there is no corresponding JCPDS. The precipitate B and precipitate C are amorphous compounds. WANG et al[13-15] re- ported that As, Sb, Bi in electrolyte interact to form arsenato antimonates that are indissoluble even in strongly acidic solution. The authors think that a kind of arsenito antimonite (precipitate A) may form in electrolyte by As(Ⅲ), Sb(Ⅲ), Sb(Ⅴ) and suggest a further study to learn the exact forming mechanism. Anyway, this technique has been successfully applied in copper electrorefining in Daye Smelting. It has the advantages of simple technique, good purification performance and low cost compared with other purification methods. Therefore, it can be expected to apply in copper electrorefining broadly.
4 Conclusions
1) The variables of n(OH-)/n(As), n(Cu)/n(As), NaOH concentration, reaction temperature and pH value have some effects on the yield of copper arsenite in the preparation of copper arsenite with As2O3. There is no copper arsenite in solution when pH value is 1.0, a kind of yellow-green compound is prepared under pH value of 4, a kind of green copper arsenite under pH value of 6 and a kind of noncrystalline copper arsenite under pH value of 10.
2) The optimum conditions of preparing copper arsenite with As2O3 are that n(OH-)/n(As) is 2?1, NaOH concentration is 1 mol/L, n(Cu)/n(As) is 2?1, pH value is 6.0 and the reaction temperature is 20 ℃. The yield of copper arsenite is as high as 98.65% under these conditions and the molar ratio of copper to arsenic is about 5?4.
3) Antimony and bismuth are removed after copper arsenite is added in copper electrolyte. The removal rates of Sb and Bi are as high as 53.85% and 53.33% respectively when copper arsenite concentration is 20 g/L. In contrast to other methods, the purification of copper electrolyte with copper arsenite has the advantages of simple technique, good purification performance and low cost.
References
[1] DUTR? V, VANDECASTEELE C. Solidification/stabilisation of hazardous arsenic containing waste from a copper refining process [J]. Journal of Hazardous Materials, 1995, 40(1): 55-68.
[2] WU Zhong. The removal of impurities As and Sb in the fluidized roasting process of zinc concentrate [J]. Inorganic Chemicals Industry, 2006(5): 43-45. (in Chinese)
[3] HONG Yu-min. Study on removal of arsenic and comprehensive utilization of flash furnace electric precipitate dust in Guixi Smelting [J]. Hydrometallurgy of China, 2003, 22(4): 208-212. (in Chinese)
[4] LEIST M, CASEY R J, CARIDI D. The management of arsenic wastes: Problems and prospects [J]. Journal of Hazardous Materials, 2000, 76(1): 125-138.
[5] RIOS-ARANA J V, WALSH E J, GARDEA-TORRESDEY J L. Assessment of arsenic and heavy metal concentrations in water and sediments of the Rio Grande at El Paso-Juarez metroplex region [J]. Environment International, 2004, 29(7): 957-971.
[6] DEBNATH R K, NUSBAR N, FITZGERALD A G. Electron beam induced chemical modification of amorphous chalcogenide–metal bilayers and its application [J]. Applied Surface Science, 2005, 243(1/4): 230-233.
[7] LIEVE H, ERIC V B. Review of disposal technologies for chromated copper arsenate (CCA) treated wood waste with detailed analyses of thermochemical conversion processes [J]. Environmental Pollution, 2005, 134(2): 301-314.
[8] RIVEROS G, UTIGARD T A. Disposal of arsenic in copper discharge slags [J]. Journal of Hazardous Materials, 2000, 77( 1/3): 241-252.
[9] WU Wei. Technological innovation and development of arsenious acid in the future [J]. Copper Engineering, 2001(3): 31-33. (in Chinese)
[10] SHIH C J, LIN C F. Arsenic contaminated site at an abandoned copper smelter plant: Waste characterization and solidification/ stabilization treatment [J]. Chemosphere, 2003, 53(7): 691-703.
[11] LIU Jing-qing. Fatal contribution of Scheele on chemistry [J]. Transaction of Zhoukou Normal College, 2002, 19(5): 39-42. (in Chinese)
[12] ZOU Qiang-ming, YANG Xin, JIN Qin-han. Pesticide and its control [J]. University Chemistry, 2004, 19(6): 1-8. (in Chinese)
[13] WANG Xue-wen. Study on the mechanism of the formation and action of arsenate antimonic acid in copper electrorefining [D]. Changsha: Central South University, 2003. (in Chinese)
[14] WANG Xue-wen, CHEN Qi-yuan, LONG Zi-ping, SU Zhong-fu, YIN Zhou-lan, ZHANG Ping-min. Application of antimony in purification of copper electrolyte [J]. The Chinese Journal of Nonferrous Metals, 2002, 12 (6): 1277-1280. (in Chinese)
[15] WANG Xue-wen, CHEN Qi-yuan, YIN Zhou-lan, XIAO Lian-sheng. Identification of arsenato antimonates in copper anode slimes [J]. Hydrometallurgy, 2006, 84(3/4): 211-217.
Corresponding author: ZHENG Ya-jie; Tel: +86-731-8836285; E-mail: ZZYYJJ01@yahoo.com.cn
(Edited by YUAN Sai-qian)