添加剂对钛酸铝陶瓷性能的影响
来源期刊:中国有色金属学报(英文版)2011年第7期
论文作者:姜澜 陈晓燕 韩国明 孟宇
文章页码:1574 - 1579
关键词:钛酸铝陶瓷;添加剂;体积密度;力学性能
Key words:aluminium titanate ceramics; additive; bulk density; mechanical properties
摘 要:研究了添加剂对钛酸铝陶瓷的体积密度、物相组成、机械强度以及抗热震性的影响。用反应烧结法制备钛酸铝陶瓷,其中添加剂为MgO、SiO2、Fe2O3及其复合添加剂。通过阿基米德排水法测试陶瓷的体积密度及气孔率,采用三点弯曲方法测试陶瓷强度,并利用热循环实验测定陶瓷抗热震性。结果表明:MgO、SiO2、Fe2O3及其复合添加剂有利于减少气孔率,提高钛酸铝陶瓷强度,增强陶瓷抗热震性。添加剂能促进烧结,形成新相,并且可以提高钛酸铝的晶格常数c。
Abstract:
The influence of some additives on bulk density, phase composition, mechanical strength and thermal shock resistance of aluminium titanate (AT) ceramics was investigated. AT ceramics with different additives of MgO, SiO2 and Fe2O3 were prepared by reaction sintering. Properties of AT ceramics were tested by using Archimedes, three-point bending and thermal cycling tests. It was found that additives of MgO, SiO2 and Fe2O3 or their compound additives are favorable to reduce the porosities of AT, enhance mechanical strength and thermal shock resistance. The role of additives can be rationalized in terms of promotion of sintering process, formation of new phases and influence on lattice constant c of AT ceramics.

JIANG Lan1, CHEN Xiao-yan2, HAN Guo-ming2, MENG Yu1
1. School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China;
2. Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
Received 8 November 2010; accepted 2 April 2011
Abstract: The influence of some additives on bulk density, phase composition, mechanical strength and thermal shock resistance of aluminium titanate (AT) ceramics was investigated. AT ceramics with different additives of MgO, SiO2 and Fe2O3 were prepared by reaction sintering. Properties of AT ceramics were tested by using Archimedes, three-point bending and thermal cycling tests. It was found that additives of MgO, SiO2 and Fe2O3 or their compound additives are favorable to reduce the porosities of AT, enhance mechanical strength and thermal shock resistance. The role of additives can be rationalized in terms of promotion of sintering process, formation of new phases and influence on lattice constant c of AT ceramics.
Key words: aluminium titanate ceramics; additive; bulk density; mechanical properties
1 Introduction
Aluminum titanate (AT) ceramic, produced by sintering equimolar mixture powders of Al2O3 and TiO2 or AT powders at the temperature of 1 500 °C, is a kind of well-known ceramic materials with low thermal expansion coefficient (9.5×10-6 °C-1), high melting point (1 860 °C) and low thermal conductivity [1]. Due to these excellent properties, AT ceramics are quite appropriate for applications requiring high thermal shock resistance or thermal insulation in the fields of steels, metallurgy, glasses, ceramics and military application [2-4]. For example, they are used as diesel engine cylinders, internal combustions, catalyst carriers and crucibles for casting and melting metal in ferrous or nonferrous metallurgy.
Nevertheless, AT has two obvious drawbacks which limit its application in industry. The first problem is its thermal instability tending to decompose into α-Al2O3 and TiO2-rutile within the temperature interval of 900- 1 200 °C [5-6]. The second disadvantage of AT ceramics is associated with its poor mechanical strength for the existence of extensive microcracks, which are mainly due to its strong anisotropy of thermal expansion [7]. In order to eliminate these two drawbacks and improve AT ceramics properties, much work has been done on doping some oxide additives into the system [8-12]. KORIM [9] found out that there was a transitional phase of Mg0.3Al1.4Ti1.3O5 in the system with MgO additive, which is useful for enhancing sintering of AT ceramics. SHI and LOW [11] reported that spodumene has an effect on densification of AT, reducing the porosity, hardness as well as thermal mechanical strength. DONG et al [12] specified the effect of both single additives (MgO, SiO2, Fe2O3 and ZrO2) and compound additives on the mechanical and thermal properties of AT ceramics, and finally pointed out that the compound additives of MgO and Fe2O3 have an excellent improvement on the stability of AT.
Although much work has been done on the role of additives in the AT ceramics, little attention is paid to the relationship between microstructure and properties of AT ceramics with different additives. In the present study, the effects of additives MgO, SiO2 and Fe2O3, and their compound additives on the microstructure and properties of AT ceramics are investigated.
2 Experimental
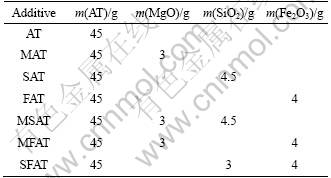
The raw materials used to fabricate Al2TiO5 (AT) ceramics were α-Al2O3 (>99%, 10 μm, Aluminum Corporation of China, Henan Branch) and TiO2 (>99%, 0.7 μm, Jinzhou Pengda Titanium Oxide Manufacturing Co. Ltd) powders on a molar ratio of 1:1. Then some additives including MgO, SiO2, Fe2O3 or their compound additives were doped into mixtures respectively to prepare different powders. In order to avoid much formation of the secondary phases during sintering process, the amount of additives was kept at a low level. The specific content of each sample is listed in Table 1.
The equimolar mixtures of α-Al2O3 and TiO2, with different additives, were thoroughly mixed through ball milling for about 10 h and then dried at 105 °C until the moisture content was below 2% (mass fraction). The compound powders were then uniaxially pressed into bars of 30 mm×5 mm×5 mm at the pressure of 200 MPa. After pressing, the samples were sintered at 1 500 °C and the calcination durations were 3 h and 6 h respectively for the purpose of comparison. The heating rate was 10 °C/min and the cooling rate was 5 °C/min, and there was a stage of 30 min at the temperature of 600 °C to volatilize the cohesive material in the samples.
Table 1 Content of raw materials and additives
Analysis of phases was performed with an X-ray diffractometer (PW3040/60) and then the lattice constant c of AT was obtained. The operation conditions were Cu Kα radiation (λ=1.540 5 ?) produced at 40 kV in 10°-90° with the current of 40 mA and the scanning rate of 8 (°)/min.
The apparent porosity and bulk density of the sintered samples were measured with the Archimedes method. The mechanical strengths of the samples were tested by three-point bending on a computer controlled electronic universal testing machine and then scanning electron microscope (SEM) was applied to observing the fracture structure of AT ceramics. Thermal cycling experiments were also carried out to examine the thermal shock resistance of AT ceramics, and three-point bending tests were performed on the samples after thermal cycling tests.
3 Results and discussion
3.1 Bulk density and apparent porosity
Figure 1 shows bulk density of samples with different additives. When sintered for 3 h, the bulk density of MAT ceramic with single additive MgO is increased greatly compared with AT ceramic without any additive. The other ceramics with other single additives seem to be no obvious change in the bulk density. However, when extending the sintering time to 6 h, it can be seen that the bulk densities of all ceramics with single additives are increased. This result indicates that the sintering duration influences the bulk density. When compound additives are doped into AT ceramics, it is evident that the bulk density of MFAT and SFAT ceramics are increased, but the bulk density of MSAT ceramic is the same as the original AT ceramic. Figure 2 exhibits the change of apparent porosity of samples with different additives. It should be noticed that the MgO-doped is more effective to reduce the apparent porosity and enhance bulk density than addition of SiO2 or Fe2O3. For example, the apparent porosity of MAT ceramic sintered for 3 h is only 8% while the apparent porosity of SAT or FAT ceramic is nearly 20%. The finding of apparent porosity is good agreement with the measurement of the bulk density except for SAT and FAT ceramics sintered for 3 h.
Bulk density and apparent porosity can be used to evaluate the influence of additives on the sintering properties of AT ceramics. The role of additives in AT ceramics commonly lies in promoting or accelerating the process of sintering. In general, additives act as active components which can reduce the energy required for sintering through forming solid solution or liquid phases as well as compounds with Al2O3-TiO2 mixtures [13]. Since different electrovalence between cations of additives and Al3+ or Ti4+ results in numerous passages for solid-state reaction and promotes the process of sintering, additives with different electrovalences and different cation radii show remarkable difference in calcination process. Thus the bulk density of ceramics is usually improved during sintering process. For example, the cation radius of Mg2+ is about 0.057 nm, which is similar to the cation radius of Al3+(0.054 nm); the cation radii of Si4+ and Fe3+ are 0.026 nm and 0.064 nm, respectively. This may be the main reason for the higher bulk density of MAT ceramics compared with SAT and FAT.
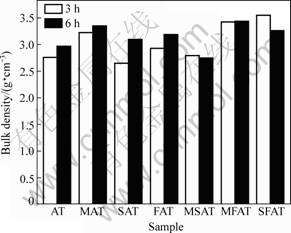
Fig. 1 Bulk density of samples with different additives
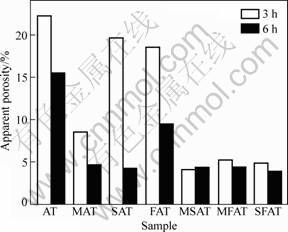
Fig. 2 Apparent porosity of samples with different additives
3.2 Phase component
X-ray analysis was used to measure the phases in the sample in order to identify the phase component variation after adding different additives to the system as well as samples without any additives. The XRD pattern of sample without additive is shown in Fig. 3. The intensity of X-ray diffraction of AT is sharp and intensive, which means that the grains are crystallized completely. It is also demonstrated that Al2TiO5 is the only new phase and almost the entire Al2O3 and TiO2 react to form AT. Sometimes since sintering time is short or the sintering temperature is low, there is a small amount of TiO2 in the phase. The patterns of sample with MgO and with compound additives SiO2 and MgO are shown in Fig. 4. It can be seen that MgAl2O4 is formed in the sample when the amount of MgO additives exceeds its solution limitation. Meanwhile, the peaks of MgAl2O4 are very low, which indicates that the amount of MgAl2O4 is quite minor. Similarly, the sample with compound additives of MgO and SiO2 also possesses little Mg2SiO4. In fact, MgAl2O4 and Mg2SiO4 are of great importance to increase the strength as well as to improve stability of AT ceramics. Meanwhile, the XRD patterns of SAT, MFAT, SFAT and FAT are shown in Fig. 5. It is noticed that with additive of SiO2, there is a little TiO2 phase remaining in the specimen SAT. For MFAT ceramic, there exists a little MgAl2O4. In MFAT and FAT, there are no other phases expect for Al2TiO5.
The lattice constant c of AT ceramics can be calculated with the aid of data from the X-ray diffraction tests, as listed in Table 2. The calculation results show that the lattice constant c of AT with additives is increased a little. According to Ref. [14], the crystal structure of AT is composed of distorted octahedral of [AlO6] and [TiO6]. The lattice constant c of AT corresponds to the height of octahedral which has a relationship with the bond angle of O—Al—O or O—Ti—O. The model of octahedral configuration is shown in Fig. 6. The greater the degree of octahedral distortion is, the worse the stability of it is. Therefore, the stability of AT can be improved by increasing lattice constant c and reducing the distortion of octahedral. In the present work, due to the replacement of cations, Mg2+ or Si4+substituting for Al3+, the lattice constant of AT ceramics is increased, producing more stable AT ceramics.
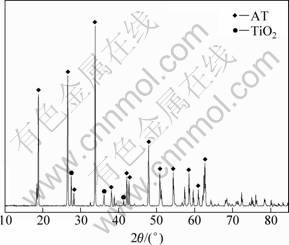
Fig. 3 XRD pattern of AT without any additive
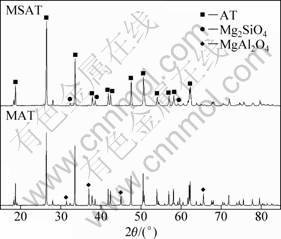
Fig. 4 XRD patterns of MSAT and MAT
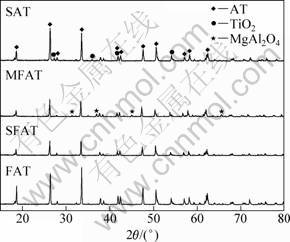
Fig. 5 XRD patterns of SAT, MFAT, SFAT and FAT
Table 2 Comparison of lattice constant c
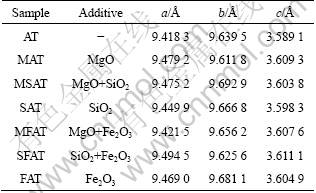
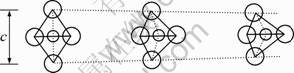
Fig. 6 Relationship between lattice constant c and distortion of octahedral [13]
3.3 Mechanical strength
Strength of AT ceramics was tested with the method of three-point bending test. From the data in Table 3, it can be seen that AT without additive only gains a minor strength about 11 MPa. By contrast, sample with additives of MgO or SiO2 as well as compound additives displays a relatively higher bending strength than AT ceramic without any additives or AT with Fe2O3. That is to say, MgO or SiO2 is beneficial to the bending strength, while Fe2O3 is detrimental to the bending strength of AT ceramics though it can be utilized as a component to increase the bulk density of AT ceramics. It has been reported that MgO substitutes Al2O3 in the sintering process and forms solid solution with Al2O3 [15]. When the amount of MgO exceeds its solid solubility limitation, the surplus MgO reacts with Al2O3 to form spinel, which lies in the boundary of AT ceramics. It is well know that spinel formed at the grain boundary hinders the crystal growth of AT, weakens the crystal domain and restrains the crack of crystal lattice. In other aspects, it is also beneficial to promote sintering and facilitate the intensification of ceramics. As for SiO2, it can react with Al2O3, forming mullite with needle-like structure. Since mullite is of high melting point, high strength and excellent resistance to creep, it is used to increase the strength of AT ceramics [16]. Unlike MgO or SiO2, the reaction mechanism of Fe2O3 with Al2O3-TiO2 system still needs to be further investigated.
Table 3 Strength and standard deviation (σ) of samples
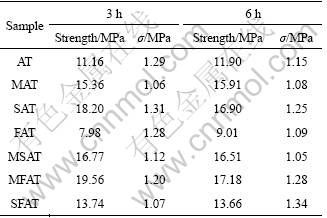
Besides, it is clearly seen that the sintering duration of 6 h or 3 h poses little difference on the bending strength of all samples in the experiments. For instance, sample SAT has strength of 18.2 MPa sintered for 3 h and 16.9 MPa for 6 h, and MFAT has strength of 19.56 MPa sintered for 3 h and 17.18 MPa for 6 h. The little decrease of mechanical strength may be mainly caused by the growth of crystal grains for longer calcination duration.
3.4 Morphology of fracture surface
In order to evaluate the effect of compound additives on the properties of ceramics and reveal the fracture behavior of AT ceramics with compound additives, some typical fracture morphologies of samples sintered for 3 h are shown in Fig. 7. For the sample MSAT, loose structure with some big cavities can be observed (Fig. 7(a)). This loose structure must be related to low bulk density of 2.79 g/cm3 and bending strength of 16.77 MPa because too many apparent pores make the bearing surface reduce and thus the material presents a low strength. For the sample MFAT, closely packed and apparent pores could hardly be seen, but some inner-crystal pores are formed (Fig. 7(b)). It is obvious that the larger bulk density of 3.42 g/cm3 and strength of 19.56 MPa are correlative with the decrease of apparent pores. Moreover, inner-crystal pores are of benefit to deactivate the main crackle and ease the stress concentration on the tip of main crackle or split the main crackle, which enhance the material strength accordingly. As for the sample SFAT, less apparent pores and inner pores are observed on the fracture surface (Fig. 7(c)). Compared with MSAT and MFAT, SFAT has the highest bulk density of 3.55 g/cm3 but the lowest strength of 13.74 MPa, which can be attributed to the addition of Fe2O3. As previously discussed, the addition of Fe2O3 is unfavorable to the mechanical strength.
3.5 Thermal shock resistance
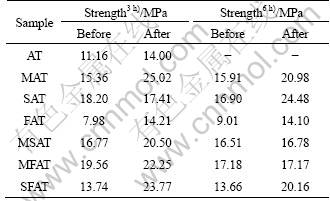
Thermal shock resistance of AT ceramics was estimated with the methods of thermal cycling at the temperatures ranging from 1 000 °C to room temperature and the specimens were quenched in the air. After 10 cycles, there is no phenomenon of crack or failure in all of the specimens. The bulk density as well as the apparent porosity shows little or no difference with samples without thermal shock tests. After thermal cycling, the three-point bending tests were performed on the specimens. It is observed that the mechanical strength is improved a little for the samples after thermal cycling, as illustrated in Table 4. But the fracture surface seems to be similar with that without thermal cycling (Fig. 8). Improvement of mechanical strength after thermal cycling should be related to microcracks which can absorb the strain energy and delay the failure of the specimens.
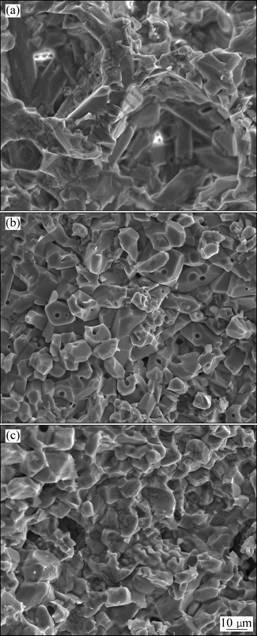
Fig. 7 Microstructures of fracture surfaces: (a) MSAT; (b) MFAT; (c) SFAT
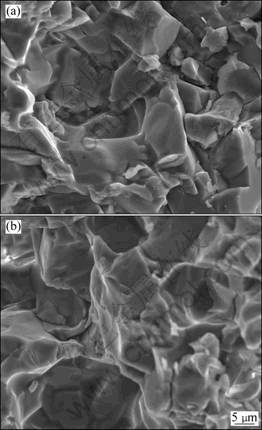
Fig. 8 Microstructures of fracture surface before (a) and after (b) thermal cycling tests
Table 4 Three-bending strength comparison before and after thermal cycling tests
4 Conclusions
1) Single additive is of great advantage to increase the bulk density and mechanical strength of AT sintered for 3 h, whereas bulk density and bending strength show little variation when the calcinations duration is 6 h.
2) Compound additives of MgO+SiO2, MgO+Fe2O3 and SiO2+Fe2O3 increase the density and strength when sintering time is 3 h. After sintering for 6 h, there is no increase in density and apparent porosity, and minor decrease of strength compared with 3 h.
3) There is minor phase of MgAl2O4 and Mg2SiO4 in the sample with MgO and MgO+SiO2, respectively as additives; the lattice constant c of AT increases with the application of additives, thus improves stability of AT.
4) AT ceramics with additives of MgO, SiO2 or compound additives show a relatively higher bending strength compared with AT without any additives and AT with Fe2O3.
5) Additives of MgO and Fe2O3 can improve the thermal shock resistance of AT ceramics due to decreasing the distortion of octahedral of AT ceramics.
References
[1] Ono T, Sawai Y, Ikimi M, Hashiba M. Acoustic emission studies of low thermal expansion aluminum-titanate ceramics strengthened by compounding mullite [J]. Ceramic International, 2007, 33: 879-882.
[2] WANG Y, YANG Y, ZHAO Y, TIAN W, BIAN H M, HE J Q. Sliding wear behaviors of in situ alumina/aluminum titanate ceramic composites [J]. Wear, 2009, 266: 1051-1057.
[3] Xie X, Sun J, Liu Y, Jiang W. Use of silica sol as a transient phase for fabrication of aluminium titanate-mullite ceramic composite [J]. Scripta Materialia, 2010, 63: 641-644.
[4] ZHAO Hao, LI Hai-jian. The preparation and application situations of low thermal expansion aluminium titanate [J]. Ceramics, 2005(7): 30-33. (in Chinese)
[5] Sobhani M, Rezaie H R, Naghizadeh R. Sol-gel synthesis of aluminum titanate (Al2TiO5) nano-particles [J]. Journal of Materials Processing Technology, 2008, 206: 282-285.
[6] Low I M, Oo Z, O’Connor B H. Effect of atmospheres on the thermal stability of aluminum titanate [J]. Physica B, 2006, 385-386: 502-504.
[7] Chen C H, Awaji H. Temperature dependence of mechanical properties of aluminum titanate ceramics [J]. Journal of the European Ceramic Society, 2007, 27: 13-18.
[8] Baghizadeh R, Rezaie H R, Golestani-fard F. The influence of composition, cooling rate and atmosphere on the synthesis and thermal stability of aluminum titanate [J]. Materials Science and Engineering B, 2009, 157: 20-25.
[9] Korim T. Effect of Mg2+-Fe3+-irons on the formation mechanism of aluminium titanate [J]. Ceramics International, 2009, 35: 1671-1675.
[10] ZHOU Lin-ping. The study of aluminium titanate by solid state fabrication and its modifying [J]. Journal of Ceramics, 2009, 30(1): 79-84. (in Chinese)
[11] SHI C G, LOW I M. Effect of spodumene additives on the sintering and densification of aluminium titanate [J]. Materials Research Bulletin, 1998, 33: 817-824.
[12] DONG Xiu-zhen, WANG Yi-ming, LI Yue. Additives’ effect on aluminium titanate ceramics [J]. China Ceramics, 2008, 44(1): 7-10. (in Chinese)
[13] MEL?NDEZ-MART?NEZ J J, JIM?NEZ-MELENDO M, DOM?NGUEZ-RODR?GUEZ A, WTTING G. High temperature mechanical behavior of aluminium titanate-mullite composites [J]. Journal of the European Ceramic Society, 2001, 21: 63-70.
[14] JIANG Wei-hui, XIAO Xing-cheng, ZHOU Jian-er, MA Guang-hua. The effects of variation of lattice constant on thermal stability of aluminium titanate ceramics [J]. Journal of Inorganic Material, 2000, 15(1): 163-168. (in Chinese)
[15] FANG Qing, ZHANG Lian-meng, SHEN Qiang, LI Ming-zhong. Aluminium titanate and its research progress [J]. Bulletin of the Chinese Ceramic Society, 2003, 22(1): 49-53. (in Chinese)
[16] FAN En-rong. Low temperature sintered mullite-titanate ceramics [J]. Refractories, 1998, 32(1): 57-58. (in Chinese)
姜 澜1,陈晓燕2,韩国明2,孟 宇1
1. 东北大学 材料与冶金学院,沈阳 110004;
2. 中国科学院 金属研究所,沈阳 110016
摘 要:研究了添加剂对钛酸铝陶瓷的体积密度、物相组成、机械强度以及抗热震性的影响。用反应烧结法制备钛酸铝陶瓷,其中添加剂为MgO、SiO2、Fe2O3及其复合添加剂。通过阿基米德排水法测试陶瓷的体积密度及气孔率,采用三点弯曲方法测试陶瓷强度,并利用热循环实验测定陶瓷抗热震性。结果表明:MgO、SiO2、Fe2O3及其复合添加剂有利于减少气孔率,提高钛酸铝陶瓷强度,增强陶瓷抗热震性。添加剂能促进烧结,形成新相,并且可以提高钛酸铝的晶格常数c。
关键词:钛酸铝陶瓷;添加剂;体积密度;力学性能
(Edited by YANG Hua)
Foundation item: Project (2009BAE80B01) supported by the Key Projects in the National Science and Technology Pillar Program During the 11th Five-Year Plan Period, China
Corresponding author: JIANG Lan; Tel: +86-24-83681325; E-mail: jiangl@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(11)60899-6

