Ag3PO4体系光催化材料的研究进展: 基础与改性
来源期刊:中国有色金属学报(英文版)2015年第1期
论文作者:马运柱 成 帆 刘文胜 王 娟 王依锴
文章页码:112 - 121
关键词:Ag3PO4体系光催化剂;电子结构;形貌控制;异质结构构筑;制备;光催化活性
Key words:Ag3PO4-based photocatalyst; electronic structure; morphology control; hetero-structure construction; preparation; photocatalytic activity
摘 要:Ag3PO4是一种高效的光催化剂,因而受到了极大的关注。其高光催化活性归功于其内禀电子结构。采用形貌控制和纳米复合体构筑的方法提高其性能和实用性。综述Ag3PO4单晶的晶体结构、性能及其基础理论,并用一些典型例子来说明提高其光催化活性的主要策略,即形貌控制和纳米复合体的构筑。
Abstract: Ag3PO4 is found to be a highly efficient photocatalyst and receives great attention. The high activity of the photocatalyst is credited to the intrinsic electronic structure. The morphology control and nano-composite fabrication are used to improve the performance and practicability. This paper reviews the structure, properties and some theoretical aspects of Ag3PO4 single crystal. Also, the major strategies, namely the morphology control and hetero-nanostructure construction, as ways to improve the performance of Ag3PO4-based photocatalysts, are summarized with the aid of some typical instances.
Trans. Nonferrous Met. Soc. China 25(2015) 112-121
Yun-zhu MA, Fan CHENG, Wen-sheng LIU, Juan WANG, Yi-kai WANG
State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China
Received 10 February 2014; accepted 12 May 2014
Abstract: Ag3PO4 is found to be a highly efficient photocatalyst and receives great attention. The high activity of the photocatalyst is credited to the intrinsic electronic structure. The morphology control and nano-composite fabrication are used to improve the performance and practicability. This paper reviews the structure, properties and some theoretical aspects of Ag3PO4 single crystal. Also, the major strategies, namely the morphology control and hetero-nanostructure construction, as ways to improve the performance of Ag3PO4-based photocatalysts, are summarized with the aid of some typical instances.
Key words: Ag3PO4-based photocatalyst; electronic structure; morphology control; hetero-structure construction; preparation; photocatalytic activity
1 Introduction
Pollutants released by both civilian and military sectors have been imposing great threats on the water quality worldwide [1-3]. Thus, on behalf of the sustainable development of human society, large amount of scientific and technological efforts are paid toward water purification. Among those strategies, heterogeneous semiconductor photocatalysis has been recognized generally as a promising green route because it paves a possible way of utilizing the solar irradiation or artificial indoor illumination to decompose contaminants [4].
Since Fujishima [5] discovered the photocatalytic phenomenon of TiO2 electrode in 1972, TiO2-based photocatalysts have been most comprehensively studied. TiO2 has the merits of low cost, strong redox capacity, relatively high activity and stability, etc [5-8]. However, due to such intrinsic limitations as wide band gap (3-3.2 eV), it is not adequate for all conditions especially under visible light irradiation [9-11]. Though doping can decrease the band gap, it will simultaneously introduce states that act as combining centers [12-16], which lowers the quantum efficiency. Thus, the development of novel visible light responsive photocatalysts has become an urgent issue.
Simple metal oxides (Fe2O3, WO3, SnO2, Bi2O3, etc) [17-20], multi-metal oxides (Bi2WO6, BiVO4, CaBi2O4, InVO4, etc) [21-24], solid solutions (ZnOx-GaN1-x, NaNbO3-AgNbO3, BiTa1-xNbxO4, etc) [25-27] have been tried in terms of obtaining the desired band gap to achieve visible absorption. Among these, Ag+-based multi-metal oxides, especially those derived by combining Ag2O and oxides of p-block elements (Ag3PO4, AgAlO2, AgGaO2, etc), were found to be promising. The improved photocatalytic performance can be credited to their similar electronic structures [28-30].
As a unique one, Ag3PO4 is so far the only compound that incorporates nonmetallic p-block specie into Ag2O. More importantly, it possesses ultra-high photocatalytic activity in O2 evolution and organic contaminant degradation [31]. Since YI et al [31] first used Ag3PO4 for photocatalysis and demonstrated the high activity, lots of researches have been devoted to Ag3PO4-based photocatalysts. Thus, a clear and complete summery of the present progress is essential. In this review, the structure, properties and some theoretical aspects of Ag3PO4 single crystal are introduced, while the major strategies, namely the morphology control and hetero-nanostructure construction, are concluded with the aid of some reliable examples.
2 Structure and properties of Ag3PO4
Ag3PO4 possesses a cubic crystal structure. The space group is 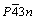 . As Fig. 1 [32] illustrated, the PO4 tetrahedron and AgO4 tetrahedron constitute the lattice [33,34]. By theoretical optimization, the cell parameters obtained are a=b=c=6.010
. As Fig. 1 [32] illustrated, the PO4 tetrahedron and AgO4 tetrahedron constitute the lattice [33,34]. By theoretical optimization, the cell parameters obtained are a=b=c=6.010 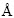 and α=β=γ=90° [35]. This nearly fits with the experimental data of a=b=c=6.026
and α=β=γ=90° [35]. This nearly fits with the experimental data of a=b=c=6.026 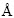 and α=β=γ=90°.
and α=β=γ=90°.
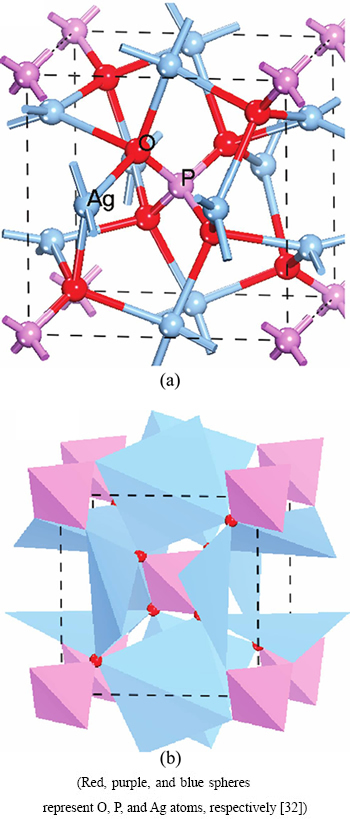
Fig. 1 Illustration of crystal structure of Ag3PO4 with ball-stick(a) and polyhedron(b)
To understand the origin of the high photocatalytic property of Ag3PO4, a comprehension of the electronic structure of the compound is needed. Experimentally, Ag3PO4 possesses an indirect band-gap of 2.36 eV as well as a direct transition of 2.43 eV, which was deduced from the ultraviolet-visible diffuse reflectance spectrum (Fig. 2) [31]. Thus, Ag3PO4 is able to absorb irradiation with a wavelength shorter than 530 nm, well extending into the visible region.
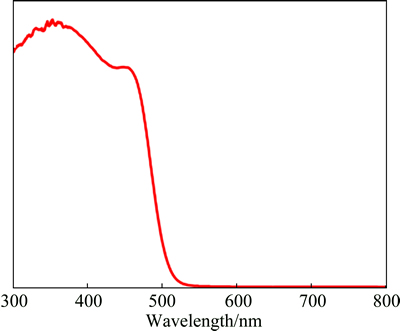
Fig. 2 Ultraviolet-visible diffusive reflectance spectrum of Ag3PO4 samples [31]
Also, theoretical studies were carried out. UMEZAWA et al [28] employed the DFT-based methods to reveal that due to the strong P—O bonds of PO4 tetrahedral units, the covalent nature of Ag—O bonds is weakened. This impairs the Ag d and O p states to blend. Thus, the d character is excluded from the conduction-band minimum (CBM), remaining highly dispersive Ag s—Ag s hybrid bands. This leads to a small effective mass of the electron, which is advantageous for the carrier transfer to surface. MA et al [33] used first-principle DFT combined with the LDA+U formalism to show that Ag3PO4 has a large dispersion of conduction band, which facilitates the separation of charge carriers. Moreover, high concentration of Ag vacancies in Ag3PO4 lattice has a significant effect on the separation of electron-hole pairs and optical absorbance in the visible-light region. In addition to the calculations performed by the above two studies, LIU et al [35] used hybrid density functional method to more precisely get the electronic structure of Ag3PO4 photocatalyst (Figs. 3 and 4). The results reveal a band gap of 2.43 eV, which agrees well with the experimental result. The conduction bands are credited to Ag 5s and 5p states, while the valence bands mainly consist of O 2p and Ag 4d states. The VBM potential was 2.67 eV (vs normal hydrogen electrode), which indicates an adequate driving force for water oxidation or pollutants degradation.
On the other hand, the photocatalytic redox mechanism is illustrated with reference to CBM, VBM and the potentials of the relevant redox pairs (Fig. 5) [31]. It is evident that Ag3PO4 is able to decompose organic pollutants or split water to produce O2 when AgNO3 is present as the sacrificial agent. Moreover, Ag3PO4 shows a much higher photocatalytic activity in comparison with WO3, BiVO4, etc [28,31,32], which implies that Ag3PO4 is potentially a highly efficient photocatalyst. Consequently, much attention has been paid to the improvement of the property of Ag3PO4 (morphology control and hetero-structure fabrication).
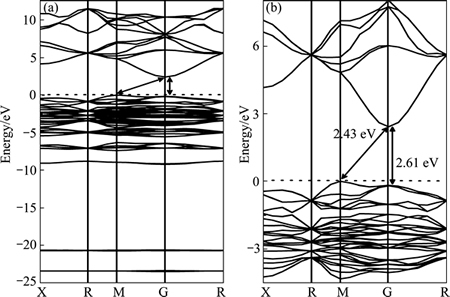
Fig. 3 Band structure of Ag3PO4 calculated using PBE0 approach (a) and magnified view of band structure near Fermi level (b) [35]
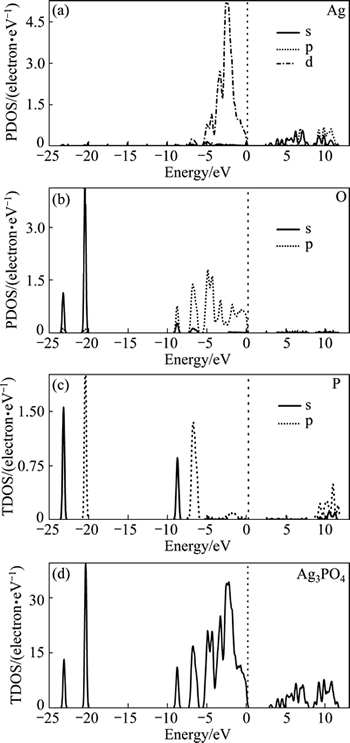
Fig. 4 TDOS and PDOS of Ag3PO4 using PBE0 approach [35]
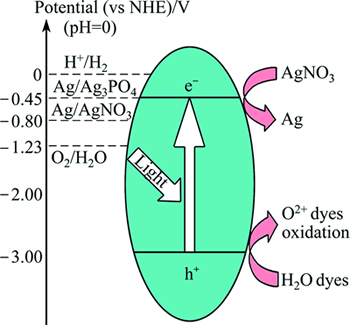
Fig. 5 Photon induced oxidation over Ag3PO4 under visible light as well as illustration of redox potentials of Ag3PO4 [31]
3 Morphology control strategy
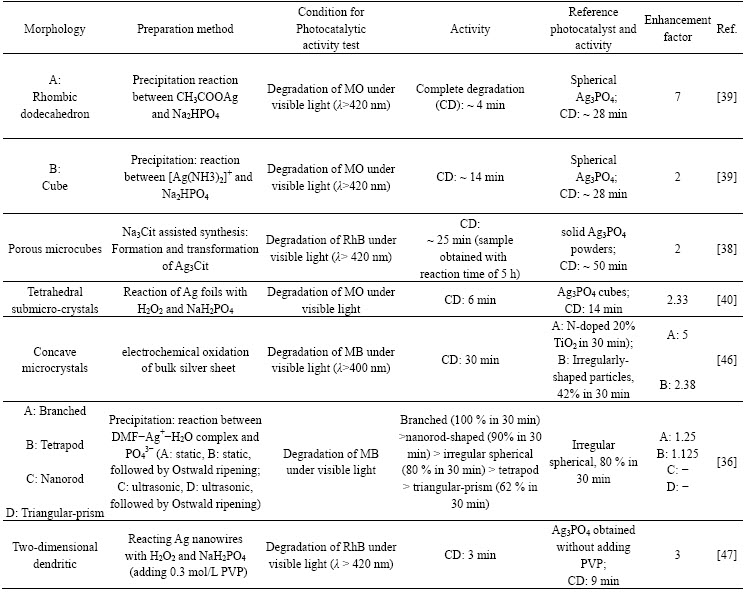
As a common sense in the field of photocatalysis, the morphology (size, shape and kind of exposed facets, etc) of the photocatalysts has an influence on the efficiency and activity [4]. This also applies, within expectation, to the Ag3PO4-based materials. Since Ag3PO4 is a promising photocatalyst, lots of researches have been conducted to the morphology tuning. Such novel shapes as branch, tetrapod, nanorod, triangular prism [36], pine tree [37] and porous microcubes [38] are successfully obtained. Generally, those preparation methods involve precipitation (with the aid of Ag+-ligant complex, organic additives or templates) [37,39], chemical or electrochemical oxidation of metallic Ag into Ag+ (subsequently captured by PO4-) [40], ligant- assisted anion exchange process [41] and hydrothermal synthesis [42] etc. Except for modulating the inner conditions of the reactions, those outside factors like ultrasound were also demonstrated to be influential to the morphology [36].
WANG et al [43] first managed to synthesize uniform tetrapod-like Ag3PO4 microcrystals (T-Ag3PO4) with a simple hydrothermal method without adding any template or surfactant. The precursor was phosphoric acid while the pH value of the reaction system was tuned by urea. By varying the amount of the urea, reaction time and temperature, the morphology of the product was successfully tuned. As the SEM images (Figs. 6(a) and (b)) shown, four arms of the tetrapod are cylindrical microrods with an average diameter of 5 μm and a length of 15-30 μm. As the XRD patterns shown (Fig. 6(c)), the intensity ratios of (110)/(200) and (222)/(321) for T-Ag3PO4 are 2.9 and 1.6, which are remarkably higher than those (0.56 and 0.9) of the irregular counterpart, respectively. From the XRD pattern, the high exposing rate of the (110) facet is demonstrated. The origin for higher intensity ratio of (110)/(200) is credited to the high surface energy of (110). The activity of the product was demonstrated in RhB degradation (Fig. 6(d)). In comparison with N-doped TiO2, the Ag3PO4 samples exhibited higher catalytic activities, while the T-Ag3PO4 possessed the highest activity. The highest activity of T-Ag3PO4 was resulted from the higher surface energy of (110) facets than those of (200) facets.
A special example, with regard to the preparation method, is worth mentioning. JIAO et al [44] fabricated various shaped Ag3PO4 microcrystals based on the heteroepitaxial growth procedure, in which different seeds were added into the reaction system before precipitation happened. This is a procedure well recognized in the field of nano fabrication, in which the nucleation and crystal growth are separated in terms of space and time [45]. Figure 7 [44] illustrates the preparation procedure as well as the SEM images of the products. Actually, considering the fact that the structure diversity of the crystal nucleus will lead to drastically different shaped products, the usage of pure single sorts of seeds are beneficial to forming pure, uniform shaped crystals as well as to obtaining the desired morphology.
Though an absence of comparison in terms of activity between individual literatures, nearly all of them showed an enhanced reaction rate with regard to the spherical counterpart and N-doped TiO2. Considering the large amount of the researches, some of the reliable and representative instances are listed in Table 1 with regard to the morphology, preparation method and photocatalytic activity.
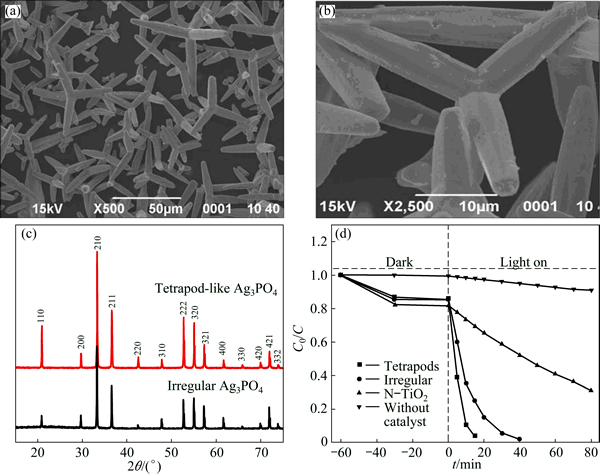
Fig. 6 SEM images of T-Ag3PO4 at different magnifications (a, b), XRD patterns of tetrapod-like and irregular Ag3PO4 (c) and degradation of RhB with tetrapod-like Ag3PO4, irregular Ag3PO4 and N-doped TiO2 under visible-light (λ>420 nm) (d) [43]
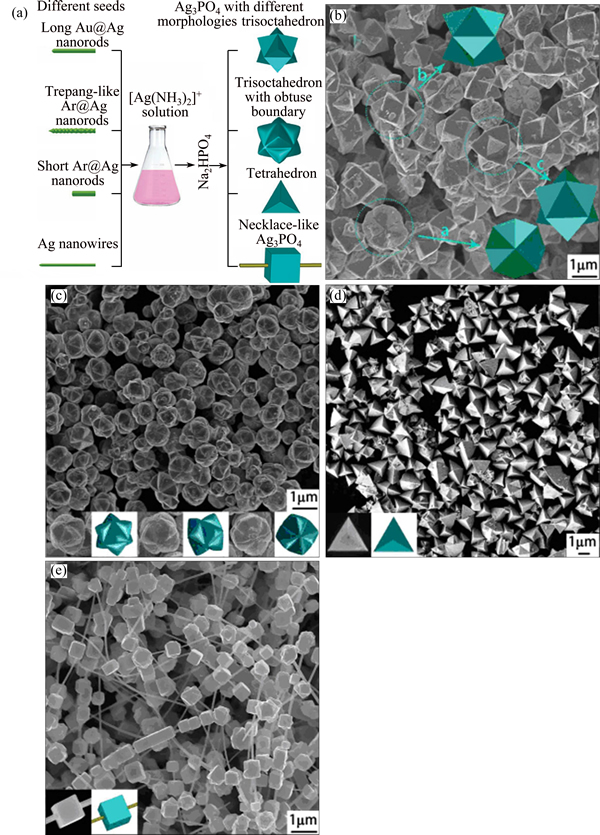
Fig. 7 Schematic illustration of growth process of Ag3PO4 with different morphologies fabricated by seed-mediated method using different seeds (a) and SEM images of four kinds of products (b-e) [44]
4 Hetero-nanostructure construction
Fabricating hetero-structure is a common strategy to enhance the activity of photocatalysts as well as to overcome some application barriers [4]. Such attempts were also made in the case of Ag3PO4. Ag3PO4-based composite photocatalysts including TiO2/Ag3PO4 (decreasing Ag content to reduce cost) [48], Fe3O4/ Ag3PO4 (magnetic separable) [49], In(OH)3/Ag3PO4 (enhancing absorption by tuning surface electric property) [50], Ag3PO4/carbon nanotube-stabilized pickering emulsion (enhancing activity by surface-chemical design of novel micro-reaction system) [51], Ag3PO4-graphene [52], Ag@(Ag2S/Ag3PO4) (facilitating migration of charge carriers) (enhancing activity via synergistic effect of Ag and Ag2S) [53], AgX/ Ag3PO4 (improving stability via core-shell structure) [54] and so forth have been successfully synthesized and studied. Each of the synthesized photocatalysts shows exclusive characters (described in the brackets behind) as well as common features shared with others like the promotion of charge carrier separation, increase of surface area and so forth. To see the literatures as a whole, one would be puzzled by the amount as well as the void of a complete comparison between the activities of the photocatalysts (variation of testing condition). To simplify, only selective instances are briefly introduced, while others are listed in Table 2.
Table 1 Preparation and photocatalytic properties of Ag3PO4 with different morphologies
HOU et al [58] prepared graphene-supported Ag3PO4/Ag/AgBr via photoassisted deposition- precipitation method. This was followed by subsequent hydrothermal treatment. Under the irradiation of visible light, the O2-evolution rate of the nano-composite was two times of that of the bare Ag3PO4 powder. Compared with unsupported Ag3PO4/Ag/AgBr, graphene supported bare Ag3PO4 and Ag/AgBr, it also performed improved activity (Fig. 8). The depletion of the conduction band electrons of Ag3PO4, downshift of the Ag3PO4 valence band influenced by silver and charge transferring onto the graphene support were responsible for the enhanced activity (Fig. 9).
Another special case, in terms of the preparation method, is the work of YU et al [59]. Necklace-like Ag3PO4– polyacrylonitrile (PAN) hetero-nanofibers were successfully fabricated through electrospinning technique (Fig. 10). The product exhibited excellent photocatalytic activities for the degradation of organic contaminants under visible light irradiation. By tuning the mass ratio (between Ag3PO4 and PAN) and applied voltage, the morphology of the product can be changed correspondently. Since electrospun polyacrylonitrile is used in clothing production, this research implies a possibility to make the clothing material photocatalytic self-cleanable.
5 Conclusion and perspective
In summery, Ag3PO4, with characteristic electronic structure, is an efficient photocatalyst, which can harness the visible light to oxidize water as well as decompose organic pollutants in aqueous solution. From the point view of quantum chemistry, Ag3PO4 is the result of inserting a nonmetallic p block element-phosphorus-into the simple narrow band gap Ag2O semiconductor. The introduction of p block elements (Al, Ga, Ge, As, and Sb, etc) into Ag2O implicates a new strategy of designing high efficiency photocatalyst. The two typical strategies, namely morphology control and hetero-structure construction, are employed to improve the activity and practicability of Ag3PO4 based photocatalysts, which are proved to be highly effective.
Table 2 Preparation and photocatalytic properties of Ag3PO4-based hetero-structures
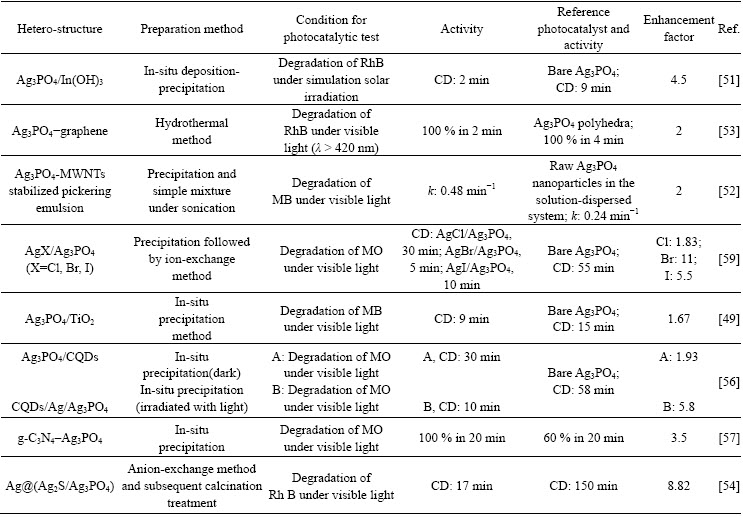
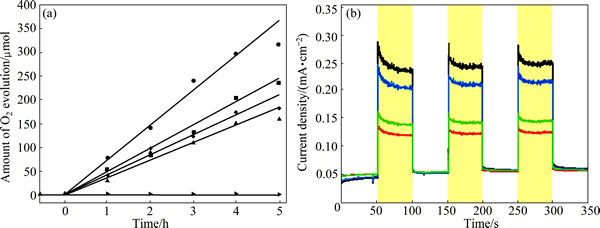
Fig. 8 Photocatalytic O2 evolution under visible light irradiation (wavelength > 420 nm) over bare Ag/AgBr, Ag3PO4, Ag3PO4/RGO, Ag3PO4/Ag/AgBr, and Ag3PO4/Ag/AgBr/RGO (from bottom to top) (a) and transient photocurrent responses of electrodes functionalized with Ag3PO4-based materials in the same order (bottom to top) as in panel (b) (Measurements proceeded in a 0.01 mol/L Na2SO4 aqueous solution under visible light irradiation (wavelength > 420 nm, I0=64 mW/cm2) at 0.5 V (vs SCE) bias [58]
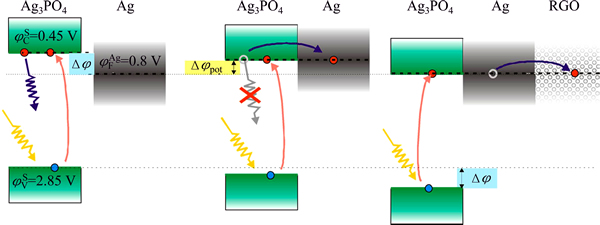
Fig. 9 Model explaining increase of activity of Ag3PO4 upon functionalization with Ag/AgBr and RGO [58]
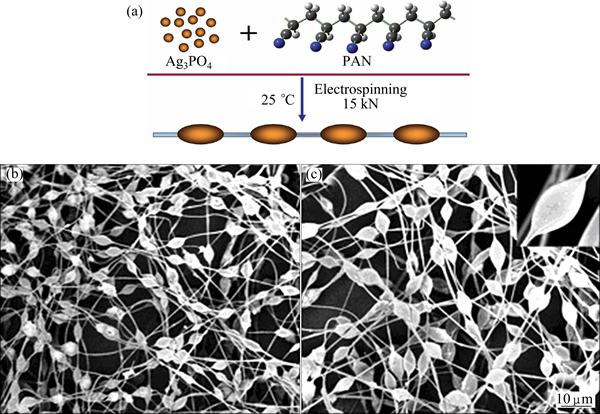
Fig. 10 Schematic illustration of formation process of Ag3PO4–PAN necklace-like nanofibers prepared by electrospinning (a) and SEM images of products (b, c) [59]
Future effort should be paid to: 1) the comparison between individual works in terms of activity of the catalysts; 2) development of novel preparation method which is environmental friendly and cost saving; 3) fabricating novel composites which possess high activity.
References
[1] HOFFMANN M R, MARTIN S T, CHOI W, BAHNEMANN D W. Environmental applications of semiconductor photocatalysis [J]. Chemical Reviews, 1995, 95(1): 69-96.
[2] SCHWARZENBACH R P, EGLI T, HOFSTETTER T B, von GUNTEN U, WEHRLI B. Global water pollution and human health [J]. Annual Review of Environment and Resources, 2010, 35: 109-136.
[3] RIFE R, THOMAS T, NORBERG D, FOURNIER R, RINKER F, BONOMO M. Chemical demilitarization: Disposing of the most hazardous wastes [J]. Environmental Progress, 1989, 8(3): 167-175.
[4] TONG H, OUYANG S, BI Y, UMEZAWA N, OSHIKIRI M, YE J. Nano-photocatalytic materials: Possibilities and challenges [J]. Advanced Materials, 2012, 24(2): 229-251.
[5] FUJISHIMA A. Electrochemical photolysis of water at a semiconductor electrode [J]. Nature, 1972, 238: 37-38.
[6] FOX M A, DULAY M T. Heterogeneous photocatalysis [J]. Chemical Reviews, 1993, 93(1): 341-357.
[7] LEGRINI O, OLIVEROS E, BRAUN A. Photochemical processes for water treatment [J]. Chemical Reviews, 1993, 93(2): 671-698.
[8] RAVELLI D, DONDI D, FAGNONI M, ALBINI A. Photocatalysis— A multi-faceted concept for green chemistry [J]. Chemical Society Reviews, 2009, 38(7): 1999-2011.
[9] ADDAMO M, AUGUGLIARO V, DI PAOLA A, GARCA L, PEZ E, LODDO V, MARC G, MOLINARI R, PALMISANO L, SCHIAVELLO M. Preparation, characterization, and photoactivity of polycrystalline nanostructured TiO2 catalystsn [J]. Journal of Physical Chemistry B, 2004, 108(10): 3303-3310.
[10] KOMINAMI H, MURAKAMI S Y, KATO J I, KERA Y, OHTANI B. Correlation between some physical properties of titanium dioxide particles and their photocatalytic activity for some probe reactions in aqueous systems [J]. Journal of Physical Chemistry B, 2002, 106(40): 10501-10507.
[11] YU J C, ZHANG L, ZHENG Z, ZHAO J. Synthesis and characterization of phosphated mesoporous titanium dioxide with high photocatalytic activity [J]. Chemistry of Materials, 2003, 15(11): 2280-2286.
[12] SAKTHIVEL S, KISCH H. Daylight photocatalysis by carbon- modified titanium dioxide [J]. Angewandte Chemie International Edition, 2003, 42(40): 4908-4911.
[13] KHAN S U, AL-SHAHRY M, INGLER W B. Efficient photochemical water splitting by a chemically modified n-TiO2 [J]. Science, 2002, 297(5590): 2243-2245.
[14] ASAHI R, MORIKAWA T, OHWAKI T, AOKI K, TAGA Y. Visible-light photocatalysis in nitrogen-doped titanium oxides [J]. Science, 2001, 293(5528): 269-271.
[15] YIN S, YAMAKI H, KOMATSU M, ZHANG Q, WANG J, TANG Q, SAITO F, SATO T. Preparation of nitrogen-doped titania with high visible light induced photocatalytic activity by mechanochemical reaction of titania and hexamethylenetetramine [J]. Journal of Materials Chemistry, 2003, 13(12): 2996-3001.
[16] LIVRAGHI S, VOTTA A, PAGANINI M C, GIAMELLO E. The nature of paramagnetic species in nitrogen doped TiO2 active in visible light photocatalysis [J]. Chemical Communications, 2005, 41(1): 498-500.
[17] CAO S W, ZHU Y J. Hierarchically nanostructured α-Fe2O3 hollow spheres: Preparation, growth mechanism, photocatalytic property, and application in water treatment [J]. Journal of Physical Chemistry C, 2008, 112(16): 6253-6257.
[18] LI L, KRISSANASAERANEE M, PATTINSON S W, STEFIK M, WIESNER U, STEINER U, EDER D. Enhanced photocatalytic properties in well-ordered mesoporous WO3 [J]. Chemical Communications, 2010, 46(40): 7620-7622.
[19] WU S, CAO H, YIN S, LIU X, ZHANG X. Amino acid-assisted hydrothermal synthesis and photocatalysis of SnO2 nanocrystals [J]. Journal of Physical Chemistry C, 2009, 113(41): 17893-17898.
[20] ANANDAN S, LEE G J, CHEN P K, FAN C, WU J J. Removal of orange II dye in water by visible light assisted photocatalytic ozonation using Bi2O3 and Au/Bi2O3 nanorods [J]. Industrial & Engineering Chemistry Research, 2010, 49(20): 9729-9737.
[21] SHANG M, WANG W, SUN S, ZHOU L, ZHANG L. Bi2WO6 nanocrystals with high photocatalytic activities under visible light [J]. Journal of Physical Chemistry C, 2008, 112(28): 10407-10411.
[22] DUNKLE S S, HELMICH R J, SUSLICK K S. BiVO4 as a visible-light photocatalyst prepared by ultrasonic spray pyrolysis [J]. Journal of Physical Chemistry C, 2009, 113(28): 11980-11983.
[23] TANG J, ZOU Z, YE J. Efficient photocatalytic decomposition of organic contaminants over CaBi2O4 under visible-light irradiation [J]. Angewandte Chemie International Edition, 2004, 43(34): 4463-4466.
[24] AI Z, ZHANG L, LEE S. Efficient visible light photocatalytic oxidation of NO on aerosol flow-synthesized nanocrystalline InVO4 hollow microspheres [J]. Journal of Physical Chemistry C, 2010, 114(43): 18594-18600.
[25] MAEDA K, TAKATA T, HARA M, SAITO N, INOUE Y, KOBAYASHI H, DOMEN K. GaN: ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting [J]. Journal of the American Chemical Society, 2005, 127(23): 8286-8287.
[26] LI G, KAKO T, WANG D, ZOU Z, YE J. Composition dependence of the photophysical and photocatalytic properties of (AgNbO3)1-x(NaNbO3)x [27] ZOU Z, ARAKAWA H, YE J. Substitution effect of Ta5+ by Nb5+ on photocatalytic, photophysical, and structural properties of BiTa1–xNbxO4 (0≤x≤1.0) [J]. Journal of Materials Research, 2002, 17(6): 1446-1454. [28] UMEZAWA N, SHUXIN O, YE J. Theoretical study of high photocatalytic performance of Ag3PO4 [J]. Physical Review B, 2011, 83(3): 035202. [29] OUYANG S, ZHANG H, LI D, YU T, YE J, ZOU Z. Electronic structure and photocatalytic characterization of a novel photocatalyst AgAlO2 [J]. Journal of Physical Chemistry B, 2006, 110(24): 11677-11682. [30] MARUYAMA Y, IRIE H, HASHIMOTO K. Visible light sensitive photocatalyst, delafossite structured α-AgGaO2 [J]. Journal of Physical Chemistry B, 2006, 110(46): 23274-23278. [31] YI Z, YE J, KIKUGAWA N, KAKO T, OUYANG S, STUART-WILLIAMS H, YANG H, CAO J, LUO W, LI Z. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation [J]. Nature Materials, 2010, 9(7): 559-564. [32] NG H, CALVO C, FAGGIANI R. A new investigation of the structure of silver orthophosphate [J]. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 1978, 34(3): 898-899. [33] MA X, LU B, LI D, SHI R, PAN C, ZHU Y. Origin of photocatalytic activation of silver orthophosphate from first-principles [J]. Journal of Physical Chemistry C, 2011, 115(11): 4680-4687. [34] MASSE R, TORDJMAN I, DURIF A. Affinement de la structure cristalline du monophosphate d'argent Ag3PO4: Existence d'une forme haute témperature [J]. Zeitschrift für Kristallographie, 1976, 144(1-6): 76-81. [35] LIU J J, FU X L, CHEN S F, ZHU Y F. Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method [J]. Applied Physics Letters, 2011, 99(19): 191903. [36] DONG P, WANG Y, LI H, LI H, MA X, HAN L. Shape-controllable synthesis and morphology-dependent photocatalytic properties of Ag3PO4 crystals [J]. J Mater Chem A, 2013, 1(15): 4651-4656. [37] LIU J K, LUO C X, WANG J D, YANG X H, ZHONG X H. Controlled synthesis of silver phosphate crystals with high photocatalytic activity and bacteriostatic activity [J]. Cryst Eng Comm, 2012, 14(24): 8714-8721. [38] LIANG Q, MA W, SHI Y, LI Z, YANG X. Hierarchical Ag3PO4 porous microcubes with enhanced photocatalytic properties synthesized with the assistance of trisodium citrate [J]. Cryst Eng Comm, 2012, 14(8): 2966-2973. [39] BI Y, OUYANG S, UMEZAWA N, CAO J, YE J. Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties [J]. Journal of the American Chemical Society, 2011, 133(17): 6490-6492. [40] HU H, JIAO Z, YU H, LU G, YE J, BI Y. Facile synthesis of tetrahedral Ag3PO4 submicro-crystals with enhanced photocatalytic properties [J]. J Mater Chem A, 2013, 1(7): 2387-2390. [41] XU Y S, ZHANG W. Morphology-controlled synthesis of Ag3PO4 microcrystals for high performance photocatalysis [J]. Cryst Eng Comm, 2013, 15(27): 5407-5411. [42] QUE R. High-yield synthesized silver orthophosphate nanowires and their application in photoswitch [J]. Frontiers of Optoelectronics in China, 2011, 4(2): 176-180. [43] WANG J, TENG F, CHEN M, XU J, SONG Y, ZHOU X. Facile synthesis of novel Ag3PO4 tetrapods and the {110} facets-dominated photocatalytic activity [J]. Cryst Eng Comm, 2012, 15(1): 39-42. [44] JIAO Z, ZHANG Y, YU H, LU G, YE J, BI Y. Concave trisoctahedral Ag3PO4 microcrystals with high-index facets and enhanced photocatalytic properties [J]. Chemical Communications, 2013, 49(6): 636-638. [45] SKRABALAK S E, XIA Y. Pushing nanocrystal synthesis toward nanomanufacturing [J]. ACS Nano, 2009, 3(1): 10-15. [46] LOU Z, HUANG B, WANG Z, ZHANG R, YANG Y, QIN X, ZHANG X, DAI Y. Fast-generation of Ag3PO4 concave microcrystals from electrochemical oxidation of bulk silver sheet [J]. Cryst Eng Comm, 2013, 15(25): 5070-5075. [47] WANG H, HE L, WANG L, HU P, GUO L, HAN X, LI J. Facile synthesis of Ag3PO4 tetrapod microcrystals with an increased percentage of exposed {110} facets and highly efficient photocatalytic properties [J]. Cryst Eng Comm, 2012, 14(24): 8342-8344. [48] YAO W, ZHANG B, HUANG C, MA C, SONG X, XU Q. Synthesis and characterization of high efficiency and stable Ag3PO4/TiO2 visible light photocatalyst for the degradation of methylene blue and rhodamine B solutions [J]. Journal of Materials Chemistry, 2012, 22(9): 4050-4055. [49] LI G, MAO L. Magnetically separable Fe3O4–Ag3PO4 sub-micrometre composite: Facile synthesis, high visible light-driven photocatalytic efficiency, and good recyclability [J]. RSC Advances, 2012, 2(12): 5108-5111. [50] GUO J, OUYANG S, ZHOU H, KAKO T, YE J. Ag3PO4/In (OH)3 composite photocatalysts with adjustable surface-electric property for efficient photodegradation of organic dyes under simulated solar-light irradiation [J]. Journal of Physical Chemistry C, 2013, 117(34): 17716-17724. [51] ZHAI W, LI G, YU P, YANG L, MAO L. Silver phosphate/carbon nanotube-stabilized pickering emulsion for highly efficient photocatalysis [J]. Journal of Physical Chemistry C, 2013, 117(29): 15183-15191. [52] YANG X, CUI H, LI Y, QIN J, ZHANG R, TANG H. Fabrication of Ag3PO4-graphene composites with highly efficient and stable visible light photocatalytic performance [J]. ACS Catalysis, 2013, 3(3): 363-369. [53] TANG J, GONG W, CAI T, XIE T, DENG C, PENG Z, DENG Q. Novel visible light responsive Ag@(Ag2S/Ag3PO4) photocatalysts: Synergistic effect between Ag and Ag2S for their enhanced photocatalytic activity [J]. RSC Advances, 2013, 3(8): 2543-2547. [54] LEE I, ALBITER M A, ZHANG Q, GE J, YIN Y, ZAERA F. New nanostructured heterogeneous catalysts with increased selectivity and stability [J]. Physical Chemistry Chemical Physics, 2011, 13(7): 2449-2456. [55] BI Y, OUYANG S, CAO J, YE J. Facile synthesis of rhombic dodecahedral AgX/Ag3PO4 (X=Cl,Br,I) heterocrystals with enhanced photocatalytic properties and stabilities [J]. Physical Chemistry Chemical Physics, 2011, 13(21): 10071-10075. [56] ZHANG H, HUANG H, MING H, LI H, ZHANG L, LIU Y, KANG Z. Carbon quantum dots/Ag3PO4 complex photocatalysts with enhanced photocatalytic activity and stability under visible light [J]. Journal of Materials Chemistry, 2012, 22(21): 10501-10506. [57] KUMAR S, SURENDAR T, BARUAH A, SHANKER V. Synthesis of a novel and stable g-C3N4–Ag3PO4 hybrid nanocomposite photocatalyst and study of the photocatalytic activity under visible light irradiation [J]. J Mater Chem A, 2013, 1(17): 5333-5340. [58] HOU Y, ZUO F, MA Q, WANG C, BARTELS L, FENG P. Ag3PO4 oxygen evolution photocatalyst employing synergistic action of Ag/AgBr nanoparticles and graphene sheets [J]. Journal of Physical Chemistry C, 2012, 116(38): 20132-20139. [59] YU H C, JIAO Z G, HU H Y, LU G X, YE J H, BI Y P. Fabrication of Ag3PO4/PAN composite nanofibers for photocatalytic applications [J]. Cryst Eng Comm, 2013, 15: 4802-4805. 马运柱,成 帆,刘文胜,王 娟,王依锴 中南大学 粉末冶金国家重点实验室,长沙 410083 摘 要:Ag3PO4是一种高效的光催化剂,因而受到了极大的关注。其高光催化活性归功于其内禀电子结构。采用形貌控制和纳米复合体构筑的方法提高其性能和实用性。综述Ag3PO4单晶的晶体结构、性能及其基础理论,并用一些典型例子来说明提高其光催化活性的主要策略,即形貌控制和纳米复合体的构筑。 关键词:Ag3PO4体系光催化剂;电子结构;形貌控制;异质结构构筑;制备;光催化活性 (Edited by Wei-ping CHEN) Corresponding author: Yun-zhu MA; Tel: +86-731-88877285; E-mail: zhuzipm@mail.csu.edu.cn DOI: 10.1016/S1003-6326(15)63585-3Ag3PO4体系光催化材料的研究进展: 基础与改性

