DOI: 10.11817/j.issn.1672-7207.2018.02.009
锂离子电池负极材料NiSi2嵌锂性质的第一性原理研究
龙朝辉1, 2, 3,丁静1, 2, 3,邓博华1, 2, 3,龚晋1, 2, 3,李小波1, 2, 3,尹付成1, 2, 3
(1. 湘潭大学 材料设计及制备技术湖南省重点实验室,湖南 湘潭,411105;
2. 湘潭大学 材料科学与工程学院,湖南 湘潭,411105;
3. 湘潭大学 装备用关键薄膜材料及应用湖南省国防科技重点实验室,湖南 湘潭,411105)
摘要:采用基于密度泛函理论的第一性原理方法研究锂离子电池负极材料NiSi2的嵌锂路径。首先计算Li嵌入NiSi2各反应的嵌锂形成能、理论质量比容量和体积膨胀率,然后研究Lix Ni8Si4 (x=0, 1, 4)的电子结构,计算其能带结构、态密度和差分电荷密度。研究结果表明:Li嵌入NiSi2最有可能的3步反应路径为12Li++12e-+7NiSi2→ Li12Si7+7NiSi,13Li++13e-+8NiSi→Li13Si4+4δ-Ni2Si和Li++e-+δ-Ni2Si→LiNi2Si;Lix Ni8Si4呈现出金属特性,Ni-Si之间形成较强的共价键,能够有效缓解嵌锂过程中的体积膨胀,稳定基体骨架,从而提高材料的循环性能。
关键词:锂离子电池;负极材料;Ni-Si合金;第一性原理
中图分类号:TG146.1 文献标志码:A 文章编号:1672-7207(2018)02-0323-07
First-principle study of Li-insertion properties of NiSi2 as anode materials for lithium-ion batteries
LONG Zhaohui1, 2, 3, DING Jing1, 2, 3, DENG Bohua1, 2, 3, GONG Jin1, 2, 3, LI Xiaobo1, 2, 3, YIN Fucheng1, 2, 3
(1. Key Laboratory of Materials Design and Preparation Technology of Hunan Province,Xiangtan University, Xiangtan 411105, China;
2. School of Materials Science and Engineering, Xiangtan University, Xiangtan 411105, China;
3. National Defense Key Laboratory of Key Film Materials & Application for Equipment,Xiangtan University, Xiangtan 411105, China)
Abstract: First-principle calculation based on the density functional theory was employed to investigate the reaction pathway of NiSi2 with Li. Li-intercalation formation energy, specific capacity and volume expansion ratio of NiSi2 as well as its lithiated products were calculated. Then, the energy band structure, density of states and deformation charge density of Lix Ni8Si4 (x=0, 1, 4) phases were discussed to study their electronic structures. The results show that the most possible reaction pathway for Li-insertion into NiSi2 is as follows: firstly, Li reacts with NiSi2 to form Li12Si7 and NiSi, then Li reacts with NiSi to produce Li13Si4 and δ-Ni2Si, and finally ternary compound LiNi2Si is formed by further lithiation of δ-Ni2Si. Lix Ni8Si4 reveals metallic feature, and there exists strong covalent bond between Ni and Si. Moreover, the strong Ni-Si covalent bond can provide a stable framework during Li intercalation process, which benefits the cyclic stability.
Key words: lithium-ion batteries; anode material; Ni -Si alloys; first-principle
锂离子电池因其具有电压高、能量密度高、循环寿命长、环境友好等优点,已广泛应用于便携式电子设备和电动汽车等领域[1]。目前,商业化的锂离子电池普遍采用石墨类碳材料作为负极材料,但这类负极材料存在比容量低(理论容量为372 mA·h/g)、易发生有机溶剂共嵌入等缺点。因此,近年来开发替代碳材料的新型负极材料成为研究热点。硅的理论储存锂容量高达4 200 mA·h/g,是极有潜力的一种高性能锂离子电池负极材料[2-4],然而,硅在嵌锂过程中体积膨胀巨大,易破坏电极结构,从而导致容量急剧衰减、电极失效。PARK等[5-6]的研究表明,通过添加惰性嵌锂金属与硅复合可有效地缓解硅在嵌锂过程中产生的体积膨胀,提高其循环性能。硅镍合金作为新型负极材料,已表现出较优异的电化学性能[7]。将镍与硅进行合金化,能保持负极材料主体结构的稳定性,缓冲脱嵌锂过程中的体积效应,提高材料的循环性能。因此,研究Si-Ni合金负极材料的嵌锂性质具有实际意义。关于Si-Ni合金嵌Li性质的研究,WEN等[8]的研究表明NiSi2首次容量达到了600 mA·h/g,当Li嵌入NiSi2时,会形成NiSi2-y化合物和非晶态的LixSiy。KIRKLIN等[9]通过第一性原理计算认为Li与NiSi2反应生成的化合物是NiSi和Li12Si7;当Li继续嵌入NiSi时,将形成Li12Si7和三元化合物LiNi2Si。而WANG等[10-11]通过高能球磨制备了NiSi合金,其结果表明Li与NiSi反应会形成LixSiy和单质Ni,此过程NiSi的容量高达1 180 mA·h/g。ZHOU等[12]采用激光沉积制备了NiSi金属间化合物薄膜,Li嵌入NiSi会形成Li22Si5和单质Ni,且NiSi的容量高达1 220 mA·h/g。但LIU等[13]通过机械球磨制取了NiSi-Si复合物,其结果表明Li嵌入NiSi负极材料会形成LixSiy和δ-Ni2Si化合物。WEN等[8-9]的研究结果表明,Li嵌入NiSi2会形成Li12Si7和NiSi。随着Li继续嵌入NiSi,KIRKLIN等[9]的计算结果表明仅需一步反应就形成了三元化合物LiNi2Si和Li-Si化合物(Li12Si7);而根据文献[10-13],Li嵌入NiSi经一步反应后并没有形成三元化合物,而是形成了LixSiy和Ni(或δ-Ni2Si)。综上所述,NiSi2的嵌Li路径目前还存在较多争议,因此,有必要对NiSi2的嵌Li路径进一步研究并阐明其嵌Li机理。本文作者采用第一性原理计算方法,计算Li嵌入NiSi2的各种可能的反应,从而推导出NiSi2最有可能的反应路径,并分析最终嵌锂产物LixNi8Si4(x=0,1,4)的电子结构,进一步揭示其嵌锂性质,以期为设计新型的Si-Ni合金负极材料提供参考依据。
1 计算方法
本文采用基于密度泛函理论的第一性原理计算软件VASP(vienna Ab-initio simulation package)[14-15]进行计算。离子和电子之间的相互作用采用全电子Bl chl投影缀加波函数(projector augmented waves,PAW)[16]方法描述。交换相关能用广义梯度近似(GGA)下的Perdew-Burke-Ernzerhof(PBE)泛函[17]进行处理。各原子的最外层电子组态分别为Li-1s22s1,Si-3s23p2和Ni-3d84s2。平面波截断能取为400 eV,以保证足够收敛,布里渊区的K点用Monkhorst-Pack方法[18]产生。K点网格数目都经过了优化,如NiSi2和LiNi2Si的K点网格设置为11×11×11。对每个结构都进行全优化(即原子位置、晶格常数、晶胞体积等),直到优化到原子间的Hellmann-Feynman力小于0.01 eV/(10-10m)为止。所有计算都是基于温度为0 K时的情况。
chl投影缀加波函数(projector augmented waves,PAW)[16]方法描述。交换相关能用广义梯度近似(GGA)下的Perdew-Burke-Ernzerhof(PBE)泛函[17]进行处理。各原子的最外层电子组态分别为Li-1s22s1,Si-3s23p2和Ni-3d84s2。平面波截断能取为400 eV,以保证足够收敛,布里渊区的K点用Monkhorst-Pack方法[18]产生。K点网格数目都经过了优化,如NiSi2和LiNi2Si的K点网格设置为11×11×11。对每个结构都进行全优化(即原子位置、晶格常数、晶胞体积等),直到优化到原子间的Hellmann-Feynman力小于0.01 eV/(10-10m)为止。所有计算都是基于温度为0 K时的情况。
理论质量比容量Ccal的计算公式如下:
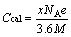 (1)
(1)
式中:x为嵌锂数目;NA为阿伏伽德罗常数;e为基本电荷的电量;M为电极材料的相对分子质量。同时,根据嵌Li前后的体积变化,定义体积膨胀率η为
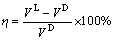 (2)
(2)
式中:VD和VL分别为嵌Li前、后主体材料的晶胞体积。平均嵌锂形成能ΔE定义为
 (3)
(3)
式中: 和
和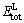 分别为嵌锂前、后各反应物和产物的原胞的总能之和;ELi为金属锂的总能,x为嵌Li数目。
分别为嵌锂前、后各反应物和产物的原胞的总能之和;ELi为金属锂的总能,x为嵌Li数目。
2 结果和分析
2.1 NiSi2的嵌Li路径
为筛选出最有可能发生的反应,得到最有可能的反应路径,可把反应的嵌Li形成能、体积膨胀率和理论比容量作为判断反应发生难易程度的主要指标[9]。热力学上,若嵌Li形成能越大,则反应越容易发生,生成的产物也更加稳定。因此,本文作者在比较各反应发生的可能性时,主要以嵌Li形成能作为判断反应发生的难易程度。此外,对于新型的负极材料而言,其理论比容量至少要与碳负极材料的比容量相当。但容量越高,嵌Li数量也越大,导致材料的体积膨胀也越大。因此,在考虑理论比容量的同时,体积膨胀率越小,反应发生的可能性越大。综上所述,若反应的嵌Li形成能越大,理论比容量越高,体积膨胀率越小,则该反应发生的可能性越大。
根据前述第一性原理计算结果[9]以及实验信息[10-13],本文假设Li嵌入NiSi经过2步反应可形成三元化合物LiNi2Si。为研究该反应路径,首先计算Li,Si和Li-Si相,Ni,LiNi2Si和Ni-Si相的总能和体积,分别如表1和表2所示。再根据式(1)~(3),得到Li与NiSi的各种可能反应的理论质量比容量、体积膨胀率和嵌Li形成能。表3~4所示分别为Li与NiSi形成LixSiy和Ni(或δ-Ni2Si)各种反应的理论质量比容量、嵌Li形成能和体积膨胀率。由表3可知:大部分反应的嵌Li形成能为负值,尽管第⑤,⑦,⑧和⑨个反应的嵌Li形成能为正值,但其值也远小于表4中各反应的嵌Li形成能。此外,表3中各反应的体积膨胀率比表4中对应的各反应的体积膨胀率要大,因此,相对于表4中的各个反应,表3中的反应不容易发生。由表4可知:第④,⑤和⑦个反应途径的嵌Li形成能较大,且比容量接近或者高于碳的理论比容量,这3个反应途径都有可能发生。其中,第⑤个反应途径的嵌Li形成能是最大的,且比容量要比第④个反应途径的大,体积膨胀率比第⑦个反应途径的要小。因此,可认为第⑤个反应途径是最有可能发生的,这与LIU等[13]的实验结果即Li嵌入NiSi形成LixSiy和δ-Ni2Si化合物相符。
随着Li继续嵌入,Li将继续与δ-Ni2Si反应。表5所示为Li嵌入δ-Ni2Si形成LiNi2Si反应的嵌Li形成能、理论比容量和体积膨胀率。由表5可知:Li嵌入δ-Ni2Si形成LiNi2Si反应的嵌Li形成能为0.373 eV,体积膨胀率仅为28.99%。该反应的理论比容量只有184.30 mA·h/g。这是因为该反应只嵌入了1个Li+,且仅是Li嵌入NiSi2反应路径中的一步,因此,该反应较容易发生。
综上所述,Li嵌入NiSi2最可能的3步反应路径如下:
表1 第一性原理计算的Li,Si和Li-Si相的总能(Etot)和体积(V)
Table 1 Total energy (Etot) and cell volume (V) of Li, Si and Li-Si phases calculated by first-principles
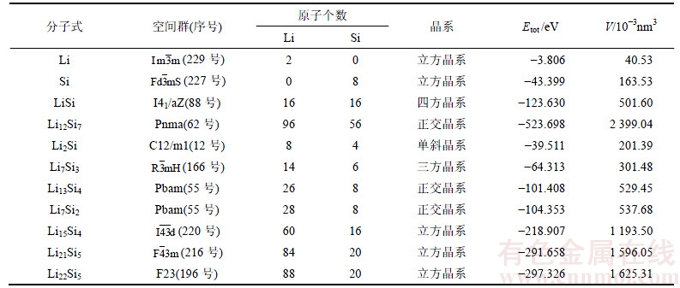
表2 第一性原理计算的Ni,LiNi2Si和Ni-Si相的总能Etot和体积V
Table 2 Total energy (Etot) and cell volume (V) of Ni, LiNi2Si and Ni-Si phases calculated by first-principles

表3 Li与NiSi反应生成LixSiy和Ni的比容量Ccal、嵌Li形成能ΔE和体积膨胀率η
Table 3 Specific capacity (Ccal), Li-intercalation formation energy (ΔE) and volume expansion ratio (η) of lithiation reaction that Li reacts with NiSi to produce LixSiy and Ni

表4 Li与NiSi反应生成LixSiy和δ-Ni2Si的比容量Ccal、嵌Li形成能ΔE和体积膨胀率η
Table 4 Specific capacity (Ccal), Li-intercalation formation energy (ΔE) and volume expansion ratio (η) for lithiation reaction that Li reacts with NiSi to produce LixSiy and δ-Ni2Si
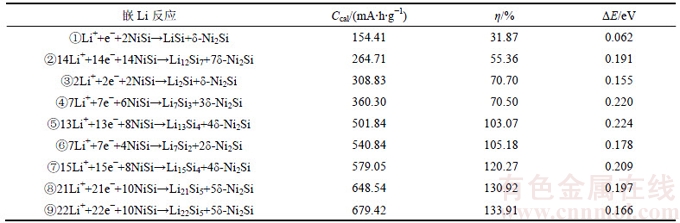
表5 Li与δ-Ni2Si反应生成LiNi2Si的比容量Ccal、嵌Li形成能ΔE和体积膨胀率η
Table 5 Specific capacity (Ccal), Li-intercalation formation energy (ΔE) and volume expansion ratio (η) of lithiation reaction for δ-Ni2Si

12Li++12e-+7NiSi2→Li12Si7+7NiSi (4)
13Li++13e-+8NiSi→Li13Si4+4δ-Ni2Si (5)
Li++e-+δ-Ni2Si→LiNi2Si (6)
2.2 δ-Ni2Si的嵌Li过程
NiSi2的第3步嵌Li反应即Li++e-+ δ-Ni2Si→ LiNi2Si,其中δ-Ni2Si的空间群为Pbnm,晶体结构模型与MgSrSi的一致;而LiNi2Si空间群为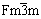 ,晶体结构模型与BiF3的相同,从δ-Ni2Si到形成LiNi2Si将会发生晶体结构的转变。为此,假设Li嵌入δ-Ni2Si的初始阶段,δ-Ni2Si吸收一定的能量后可能先转变为反萤石结构,该过程的体积膨胀率为13.656%。随着Li继续嵌入,Li将继续与反萤石结构的Ni2Si反应,并最终形成LiNi2Si。反萤石结构的Ni2Si晶胞中包含8个Ni原子和4个Si原子。图1所示为LixNi8Si4(x=0,1,4)的晶体结构模型。计算时,假设Li嵌入到反萤石结构的Ni2Si(Ni8Si4)晶胞中,生成嵌Li合金相LixNi8Si,此过程不改变晶胞结构[19]。该嵌Li过程的反应式如下:
,晶体结构模型与BiF3的相同,从δ-Ni2Si到形成LiNi2Si将会发生晶体结构的转变。为此,假设Li嵌入δ-Ni2Si的初始阶段,δ-Ni2Si吸收一定的能量后可能先转变为反萤石结构,该过程的体积膨胀率为13.656%。随着Li继续嵌入,Li将继续与反萤石结构的Ni2Si反应,并最终形成LiNi2Si。反萤石结构的Ni2Si晶胞中包含8个Ni原子和4个Si原子。图1所示为LixNi8Si4(x=0,1,4)的晶体结构模型。计算时,假设Li嵌入到反萤石结构的Ni2Si(Ni8Si4)晶胞中,生成嵌Li合金相LixNi8Si,此过程不改变晶胞结构[19]。该嵌Li过程的反应式如下:
xLi+ +xe- +Ni8Si4→LixNi8Si4(0<x≤4) (7)
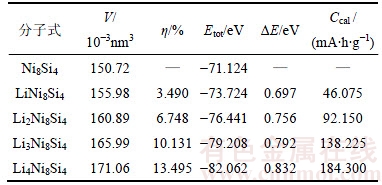
为进一步研究Ni8Si4的嵌Li过程,计算各个嵌Li合金相的电化学参数,如表6所示。由表6可知:嵌Li形成能随着Li嵌入量的增大而增大,其平均值约为0.76 eV。在热力学上,嵌Li形成能越大,嵌Li反应越容易发生,生成的产物也越稳定。此外,即使Ni8Si4所有的八面体间隙都被Li所占据,其体积膨胀率也只有13.495%,因此,Li能够较容易地嵌入Ni8Si4形成Li4Ni8Si4。
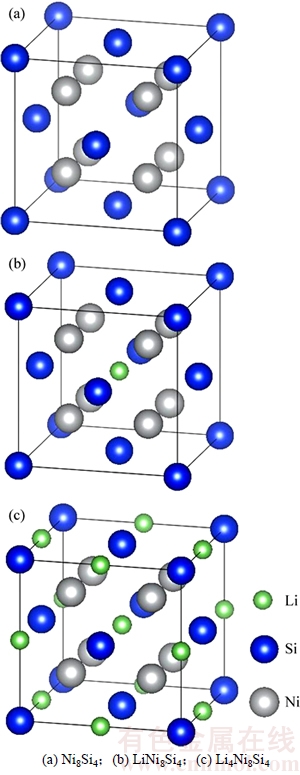
图1 Lix Ni8Si4的晶体结构
Fig. 1 Crystal structures of Lix Ni8Si4
表6 Li xNi8Si4相的晶胞体积V、体积膨胀率η、总能Etot、嵌Li形成能ΔE和比容量Ccal
Table 6 Cell volume (V), volume expansion ratio (η), total energy (Etot), Li-intercalation formation energy (ΔE) and specific capacity (Ccal) for LixNi8Si4 phases
2.3 LixNi8Si4(x=0,1,4)的电子结构
图2所示为反萤石结构的Ni2Si在嵌锂过程中生成的3种典型合金相LixNi8Si4(x=0,1,4)的能带结构。由图2可知三者的能带结构大体相似,能带均在Fermi能级附近相互交错并穿越Fermi能级,且均表现出金属性质,这说明Li嵌入Ni8Si4前后没有发生导电性质的相变。
图3所示为LixNi8Si4(x=0,1,4)合金相的总态密度(TDOS)图和分波态密度(PDOS)图。Fermi能级处的态密度不为0 eV,也正好说明了它们呈现出金属性质,这与能带结构分析结果一致。由图3(a)可知:在Fermi能级以下即-12~-8 eV之间的态密度主要由Si-3s电子贡献;在-6~0 eV之间的态密度主要由Ni-3d电子贡献,而Si-3p电子的贡献相对较小,Fermi能级处的态密度N(EF)为4.855 eV-1。此时,Ni-Si原子间的共价作用主要由Ni-3d与Si-3p轨道的电子杂化引起。由图3(b)可知:Li原子对态密度的贡献主要表现在Fermi能级附近,其余与Ni8Si4的态密度(DOS)基本相似。由于Li的嵌入,Si的电荷向Ni转移,且Si-3p与Li-2s电子相互作用,使得Si原子对态密度的贡献下降。LiNi8Si4在Fermi能级处的态密度,N(EF)为 4.483 eV-1,表明体系中金属性略有减弱。由图3(c)可知:当Li占据Ni8Si4所有的八面体间隙位置形成Li4Ni8Si4时,Li和Si原子对Fermi能级处总态密度贡献有所增加,而Ni原子的贡献有所减小,N(EF)为3.950 eV-1,这表明此时体系的金属性有所下降。N(EF)变小,是因为随着Li嵌入,更多电子进入Ni8Si4的结构中,其中一些电子由Si转移到了Ni上,并占据更多的Ni-3d轨道,使得Ni-3d态转移到更低的能量区域。此外,N(EF)越小,对应的材料越稳定[20]。N(EF)变小也表明Ni8Si4经LiNi8Si4到Li4Ni8Si4的稳定性有所提高,这与嵌Li形成能增加、生成的材料越稳定的结论相符合。总而言之,虽然N(EF)有所变小,但仍然远大于0 eV。因此,LixNi8Si4仍然呈现良好的金属性质。
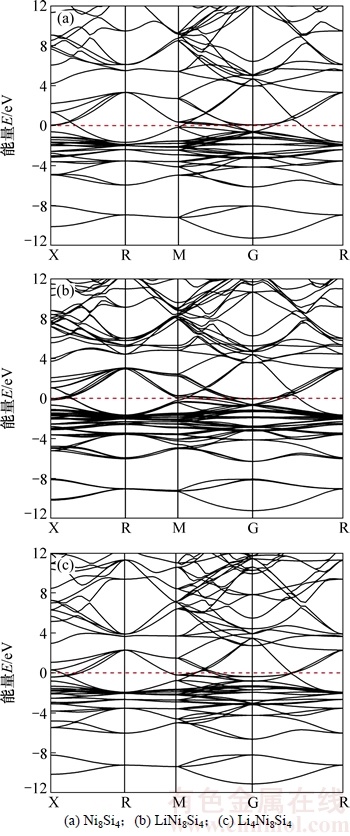
图2 典型合金相的能带结构
Fig. 2 Energy band structures of typical alloy phases
为直观地理解原子间的成键性质,进一步计算各嵌Li相的差分电荷密度。图4所示为典型合金相的(1 1 0)面差分电荷密度。由图4(a)可知:Ni-Si原子之间的电荷密度差异较大且呈一定的方向性,说明Ni-Si之间主要呈现共价键的特征。由图4(b)和(c)可知:Li-Si之间主要以共价键结合,且随着嵌Li量增加,共价作用稍有增强;而Ni-Si之间仍然呈现出较强的共价键。正是由于Ni-Si之间存在较强的共价键,使得主体结构框架保持稳定,从而提高了材料的循环性能。
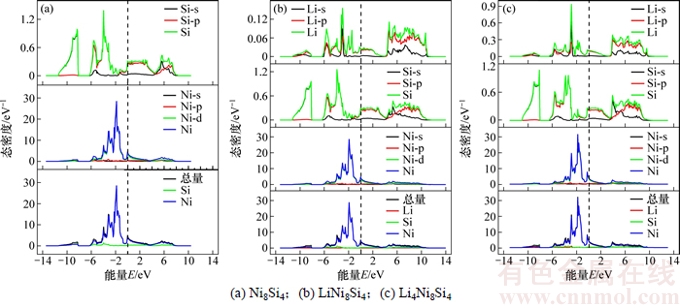
图3 典型合金相的电子态密度
Fig. 3 Density of states of typical alloy phases
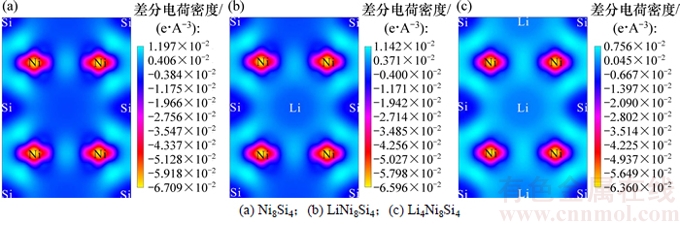
图4 典型合金相的(1 1 0)面差分电荷密度
Fig. 4 Charge differential density of (1 1 0) plane in typical alloy phases
3 结论
1) Li嵌入NiSi2最有可能的3步反应路径为:
12Li++12e-+7NiSi2→Li12Si7+7NiSi;
13Li++13e-+8NiSi→Li13Si4+4δ-Ni2Si;
Li++e-+δ-Ni2Si→LiNi2Si。
2) Li嵌入反萤石结构的Ni2Si(Ni8Si4)的平均嵌Li形成能约为0.76 eV,表明Li能较容易嵌入到Ni8Si4。Li嵌入Ni8Si4前后的化合物没有导电性质的相变,都呈现出良好的金属性质;Ni-Si原子之间以较强的共价键结合。这种较强的共价键能够提高主体结构稳定性,进而改善材料的循环性能。
参考文献:
[1] LI Aiyu, WU Shunqing, YANG Yong, et al. Structural and electronic properties of Li-ion battery cathode material MoF3 from first-principles[J]. Journal of Solid State Chemistry, 2015, 227: 25-29.
[2] CHANDRASEKAEA R, MAGASINSKI A, YUSHIN G, et al. Analysis of lithium insertion/deinsertion in a silicon electrode particle at room temperature[J]. Journal of the Electrochemical Society, 2010, 157(10): A1139-A1151.
[3] CUI Zhiwei, GAO Feng, CUI Zhihua, et al. A second nearest-neighbor embedded atom method interatomic potential for Li-Si alloys[J]. Journal of Power Sources, 2012, 207(6): 150-159.
[4] YIN Yaxia, SEN Xin, WAN Lijun, et al. Electrospray synthesis of silicon/carbon nanoporous microspheres as improved anode materials for lithium-ion batteries[J]. The Journal of Physical Chemistry C, 2011, 115(29): 14148-14154.
[5] PARK C M, KIM J H, KIM H, et al. Li-alloy based anode materials for Li secondary batteries[J]. Chemical Society Reviews, 2010, 39(8): 3115-3141.
[6] FLEISCHAUER M D, TOPPLE J M, DAHN J R. Combinatorial Investigations of SiM (M=Cr+Ni, Fe, Mn) thin film negative electrode materials[J]. Electrochemical and Solid-State Letters, 2005, 8(2): A137-A140.
[7] KIM H, IM D, DOO S G. Electrochemical properties of Ni-based inert phases incorporated Si/graphite composite anode[J]. Journal of Power Sources, 2007, 174(2): 588-591.
[8] WEN Zhongsheng, JI Shijun, SUN Juncai., et al. Mechanism of lithium insertion into NiSi2 anode material for lithium ion batteries[J]. Rare Metals, 2006, 25(6): 77-81.
[9] KIRKLIN S, MEREDING B, WOLVERTON C. High-throughput computational screening of new Li-ion battery anode materials[J]. Advanced Energy Materials, 2013, 3(2): 252-262.
[10] WANG Guoxiu, SUN Li, BRADHURST D H, et al. Innovative nanosize lithium storage alloys with silica as active centre[J]. Journal of Power Sources, 2000, 88(2): 278-281.
[11] WANG Guoxiu, SUN Li, BRADHURST D H, et al. Nanocrystalline NiSi alloy as an anode material for lithium-ion batteries[J]. Journal of Alloys and Compounds, 2000, 306(1/2): 249-252.
[12] ZHOU Yongning, LI Wenjing, CHEN Huajun, et al. Nanostructured NiSi thin films as a new anode material for lithium ion batteries[J]. Electrochemistry Communications, 2011, 13(13): 546-549.
[13] LIU Weiren, WU N L, SHIEH D T, et al. Synthesis and characterization of nanoporous NiSi-Si composite anode for lithium-ion batteries[J]. Journal of the Electrochemical Society, 2007, 154(2): A97-A102.
[14] KRESSE G, FURTHMULLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Computational Materials Science, 1996, 6(1): 15-50.
[15] KRESSE G, FURTHMULLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Physical Review B, 1996, 54(16): 11169.
[16] KRESSE G, JOUBERTJ D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B, 1999, 59(3): 1758-1775.
[17] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865.
[18] MONHORST H J, PACK J D. Special points for Brillouin-zone integrations[J]. Physical Review B, 1976, 13(12): 5188.
[19] LEE J W, ANGUCHAMY Y K, POPOV B N. Simulation of charge-discharge cycling of lithium-ion batteries under low-earth-orbit conditions[J]. Journal of Power Sources, 2006, 162(2): 1395-1400.
[20] YU Chun, LIU Junyan, LU Hao, et al. First-principles investigation of the structural and electronic properties of Cu6-xNixSn5(x=0, 1, 2) intermetallic compounds[J]. Intermetallics, 2007, 15(11): 1471-1478.
(编辑 伍锦花)
收稿日期:2017-03-12;修回日期:2017-05-16
基金项目(Foundation item):国家自然科学基金资助项目(51201146);湖南省教育厅优秀青年教师基金资助项目(15B230)(Project(51201146) supported by the National Natural Science Foundation of China; Project(15B230) supported by Science Foundation for the Excellent Youth Scholars of Educational Commission of Hunan Province)
通信作者:龙朝辉,博士,副教授,从事材料热力学与动力学、锂离子电池电极材料、第一性原理计算等研究;E-mail:zhlong@xtu.edu.cn