DOI:10.19476/j.ysxb.1004.0609.2017.07.19
氯化铅在碳酸氢铵溶液中的物相转化机理
李 云1,陈永明1,薛浩天2,唐朝波1,杨声海1,唐谟堂1
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 青海省环境科学研究设计院,西宁 810000)
摘 要:详细研究氯化铅在碳酸氢铵溶液中的物相转化机理,对比分析同一条件下不同转化剂的转化脱氯效果,转化脱氯效果由大到小依次为NH4HCO3、Na2CO3、(NH4)2CO3、K2CO3;得出NH4HCO3作为转化剂时的最佳转化条件:溶液初始pH=10.0、NH4HCO3浓度1.68 mol/L、反应温度50 ℃、反应时间120 min;PbCl2在转化过程中会经历PbCl2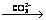 Pb2Cl2(CO3)
Pb2Cl2(CO3)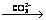 PbCO3的转化过程,在最佳工艺条件下转化率可达99%以上,转化产物为纯净的PbCO3。通过不同转化阶段得到的产物的物相分析,确定PbCl2在NH4HCO3溶液中转化为PbCO3的机理、途径及影响机制。转化体系的pH值是影响转化率、转化途径及最终产物物相组成的最显著因素,而转化剂浓度、转化时间和温度,只会对PbCl2脱氯速率造成影响,并不会影响PbCl2的转化途径和最终产物组成。
PbCO3的转化过程,在最佳工艺条件下转化率可达99%以上,转化产物为纯净的PbCO3。通过不同转化阶段得到的产物的物相分析,确定PbCl2在NH4HCO3溶液中转化为PbCO3的机理、途径及影响机制。转化体系的pH值是影响转化率、转化途径及最终产物物相组成的最显著因素,而转化剂浓度、转化时间和温度,只会对PbCl2脱氯速率造成影响,并不会影响PbCl2的转化途径和最终产物组成。
关键字:氯化铅;碳酸氢铵;物相转化机理;脱氯率;转化产物
文章编号:1004-0609(2017)-07-1479-06 中图分类号:TF812 文献标志码:A
铅阳极泥氯化浸出[1-2],粗铋氯化精炼除铅[3]和熔盐电解[2, 4]等都会产出氯化铅渣。由于氯化铅的低熔点、高挥发性、易潮解的特点,在后续火法熔炼过程中,氯化铅会大量挥发进入烟气,不但大大降低铅的直收率,还会造成后续收尘工序布袋粘结、设备腐蚀等诸多问题[3]。因此,现有生产实践中,在入炉前,对含氯化铅物料都会进行脱氯转化处理。
目前,在铅化合物物相转化研究方面,主要集中在废铅蓄电池胶泥(PbSO4)转化脱硫[5-7]方面。比如MORACHEVSKII等[8]和ARAI等[9]利用Na2CO3作为转化剂,分别从热力学和动力学方面系统研究PbSO4转化为PbCO3的转化机理和主要影响条件,最终得到转化剂浓度、溶液pH值以及反应温度,对转化效率和产物的影响最大。此外,GONG等[10-11]系统研究了PbSO4物相转化的动力学机理,并得到以下结论:PbSO4与Na2CO3之间的物相转化反应遵循扩散控制收缩核模型,转化率随Na2CO3浓度和反应温度的提高而提高,溶液pH对转化机理和转化产物的影响非常显著。此外,在硫化铅脱硫转化方面,也有不少研究,比如LU等[12]利用(NH4)2CO3作为转化剂,详细研究含PbS废料脱硫转化工艺,得到最佳工艺条件,并在此条件下,转化率可以达到90%以上,其转化产物为PbCO3。
在处理含PbCl2废料方面,也引起不少国内外学者关注并研究[13]。比如,王淼生[14]和LIN等[15]提出的碱性转化工艺,即先用NaOH对PbCl2进行碱性处理,再利用NH4HCO3或者(NH4)2CO3作为转化剂,对碱式氯化铅进行脱氯转化。但是,工艺流程繁琐,且成本高,产物也不是纯净的碳酸铅。唐谟堂等[16]用Na2CO3作转化剂,对铅锑精矿的氯化浸出法进行了转化研究,研究结果表明,PbCl2和PbSO4的转化率分别达到了99.83%和80%。
本文作者以纯氯化铅为原料,详细地研究了其在不同转化剂作用下的物相转化过程及产物,深入研究氯化铅在不同初始pH值、转化剂浓度、转化温度和时间作用下的物相转化工艺及机理,对冶金行业含氯化铅物料的脱氯转化处置具有重要理论指导意义。
1 实验
1.1 实验原料及试剂
实验所用的氯化铅、氨水和碳酸氢铵等转化剂均为分析纯级,氯化铅经过研磨、筛分后取105~125 μm粒径用于本试验,所用反应溶液均由去离子水按比例配制。
1.2 实验方法
试验称取一定量的转化剂溶于去离子水中,移入三口平底烧瓶中,烧瓶置于恒温水浴槽内控制反应温度和搅拌速度(150 r/min);通过添加氢氧化钠或者氨水调节溶液pH至预设值,加入一定量的氯化铅,反应一定时间,待试验完毕后,对产物进行液固分离,并对滤液和固体产物进行分析,滤液可通过蒸发、浓缩、结晶的方法回收NH4Cl晶体。
利用银量法对产物滤液中的Cl-进行化学定量分析;固体产物在55 ℃下,真空干燥12h,充分脱去水分后,利用X-ray Fluorescence (XRF, Panalytical B V, Axios, PW4400/40)对固体产物中的化学组成进行半定量分析,利用X-ray Diffraction (XRD, Rigaku 3014, Cu-Kα-radiation, λ=1.54 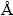 ) 对产物中的物相组成进行物相分析,确定不同条件及不同反应阶段下PbCl2的物相转化规律。由于氯化铅和碳酸铅都属于难溶性物质,所以直接计算铅的转化率非常困难,故而通过计算氯化铅的脱氯率,来表征转化反应进行的程度,具体计算方法如下:
) 对产物中的物相组成进行物相分析,确定不同条件及不同反应阶段下PbCl2的物相转化规律。由于氯化铅和碳酸铅都属于难溶性物质,所以直接计算铅的转化率非常困难,故而通过计算氯化铅的脱氯率,来表征转化反应进行的程度,具体计算方法如下:
 (1)
(1)
式中:wCl为固体转化产物中Cl含量;ms为固体转化产物质量;wi,Cl为初始PbCl2原料中Cl含量。
2 结果与讨论
2.1 转化剂种类的影响
在转化剂用量过剩系数α=1.3(即理论用量的1.3倍,1.56 mol/L)、温度50 ℃、时间180 min的固定条件下,调节体系初始pH为12.0,详细考察转化剂种类对氯化铅转化脱氯过程的影响,结果如表1所列。
表1 采用不同转化剂所得转化产物分析结果
Table 1 Chemical composition of products prepared by different conversion agents

由表1可看出,在4种转化剂中,K2CO3的脱氯效果最差,转化产物含氯量高达6.87%,脱氯率仅为72.17%;NH4HCO3的脱氯效果最好,转化产物中含氯量小于0.5%,脱氯率高达96.11%;(NH4)2CO3和Na2CO3脱氯效果相似,分别达到81.58%和82.49%。为进一步分析不同转化剂作用下的转化机理,对所得转化产物进行XRD表征,XRD谱如图1所示。由图1中可以看出,采用不同转化剂,所得转化产物的物相组成差异明显。以K2CO3为转化剂时,转化产物为PbOHCl,这与表1中脱氯率数据相吻合,说明氯根离子并未完全脱除;采用Na2CO3作为转化剂时,转化产物主要为NaPb2(CO3)2OH和PbCl2(CO3),还夹杂有少量的PbOHCl和PbCO3,脱氯效果仍不显著。以 (NH4)2CO3 和NH4HCO3为转化剂时,转化产物主要为NaPb2(CO3)2OH,这是由于在调节体系初始pH=12值时,加入了NaOH,有部分过剩的NaOH与PbCO3物相结合所形成的新物相,说明溶液的pH值及pH调节剂种类对最终转化产物组成有一定影响,pH值过高,会使得转化产物纯度降低。同时,在以(NH4)2CO3为转化剂时,转化产物内还夹杂有少量PbCl2(CO3)、PbOHCl和独立的PbCO3物相,说明以(NH4)2CO3为转化剂的转化脱氯过程仍不彻底,而以NH4HCO3为转化剂,转化产物组成稳定、单一,脱氯效果好。综上所述可知,在同一工艺条件下,转化剂的转化脱氯效果由大到小依次为NH4HCO3、Na2CO3、(NH4)2CO3、K2CO3,综合考虑转化剂来源及其脱氯效果,在后续试验中,选择碳酸氢铵作为最佳转化剂。

图1 不同转化剂下所得产物的XRD谱
Fig. 1 XRD patterns of products in different conversion agents
2.2 初始pH的影响
在NH4HCO3浓度1.56 mol/L(即过剩1.3倍)、温度50 ℃、时间180 min的固定条件下,考察初始pH值对PbCl2物相转化过程的影响,为避免引入NaOH污染转化产物,试验通过添加氨水调节溶液初始pH值,结果如图2所示。由图2可知,不同pH值下,试验结果差异非常明显,当溶液中的pH值由8.0提高至10.0时,脱氯率从23.35%快速提高至98%;pH值继续从10.0升高到12,脱氯率稳定在98%左右不再提高。说明此时PbCl2的脱氯转化已达极限。
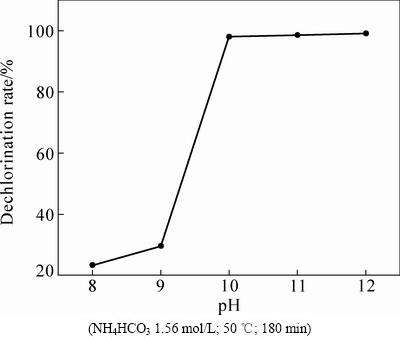
图2 初始pH值对脱氯率的影响
Fig. 2 Effect of initial pH value on dechlorination rate
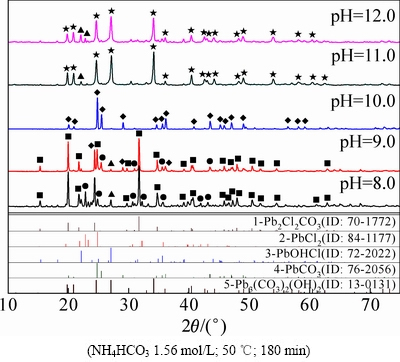
图3 不同初始pH值下所得转化产物的XRD谱
Fig. 3 XRD patterns of products at different initial pH values
图3所示为不同pH值下转化产物的XRD谱。由图3可知,当体系的pH值较低(8.0~9.0)时,由于不能保证足够的OH-与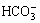 反应生成
反应生成 ,如反应(2)所示,造成溶液中
,如反应(2)所示,造成溶液中 浓度不足,
浓度不足, 离子在反应溶液中的扩散速度慢,从而形成Pb2Cl2(CO3)中间物相,如反应(3)所示,脱氯反应不充分。同时,OH-也会按反应(4)与PbCl2反应生成PbOHCl,说明碱性体系下,OH-离子也具有一定的转化剂作用,促进PbCl2转化为中间产物PbOHCl,但中间产物继续转化生成最终产物PbCO3仍需依靠体系内
离子在反应溶液中的扩散速度慢,从而形成Pb2Cl2(CO3)中间物相,如反应(3)所示,脱氯反应不充分。同时,OH-也会按反应(4)与PbCl2反应生成PbOHCl,说明碱性体系下,OH-离子也具有一定的转化剂作用,促进PbCl2转化为中间产物PbOHCl,但中间产物继续转化生成最终产物PbCO3仍需依靠体系内 的转化作用,如反应(5)所示。此结果与相关学者的研究[9, 17-19]结果基本吻合。
的转化作用,如反应(5)所示。此结果与相关学者的研究[9, 17-19]结果基本吻合。
HCO3-(aq)+OH-(aq) 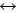 CO32-(aq)+H2O(aq) (2)
CO32-(aq)+H2O(aq) (2)
2PbCl2(s)+CO32-(aq)→Pb2Cl2(CO3)(s)+2Cl-(aq) (3)
PbCl2(s)+OH-(aq)→PbOHCl(s)+Cl-(aq) (4)
PbOHCl(s)+CO32-(aq)→PbCO3(s)+Cl-(aq)+OH-(aq) (5)
PbCl2(s)+CO32-(aq)→PbCO3(s)+2Cl-(aq) (6)
3PbCO3(s)+2OH-(aq)→Pb3(CO3)2(OH)2(s)+CO32-(aq) (7)
溶液的pH值升高,反应(2)向右进行趋势增大,溶液中 浓度不断上升,脱氯效果增强,如反应(6)所示,
浓度不断上升,脱氯效果增强,如反应(6)所示, 可将PbCl2 一步转化得到PbCO3,PbCO3物相增多,成为反应的主要产物和最终产物。当溶液中pH值达到10时,脱氯率已高达98%,说明氯化铅物相转化反应在此条件下可以进行地非常彻底。如果溶液pH值继续升高,会造成溶液中OH-浓度过高,并参与PbCl2与
可将PbCl2 一步转化得到PbCO3,PbCO3物相增多,成为反应的主要产物和最终产物。当溶液中pH值达到10时,脱氯率已高达98%,说明氯化铅物相转化反应在此条件下可以进行地非常彻底。如果溶液pH值继续升高,会造成溶液中OH-浓度过高,并参与PbCl2与 的反应过程,如反应(7)所示,从而得到Pb3(CO3)2(OH)2和PbCO3的混合物。因此,溶液的初始pH值是影响PbCl2脱氯转化效果的一个关键因素,pH值过低或过高,会引起PbCl2转化不充分或目标产物的污染而造成转化产物PbCO3纯度的降低。
的反应过程,如反应(7)所示,从而得到Pb3(CO3)2(OH)2和PbCO3的混合物。因此,溶液的初始pH值是影响PbCl2脱氯转化效果的一个关键因素,pH值过低或过高,会引起PbCl2转化不充分或目标产物的污染而造成转化产物PbCO3纯度的降低。
2.3 NH4HCO3浓度的影响
固定其他的反应条件,通过改变溶液中NH4HCO3浓度,考察其对氯化铅物相转化规律的影响,结果如图4所示。由图4可知,当NH4HCO3浓度由1.20 mol/L增加到1.68 mol/L时,脱氯率由96.36%提高至98.46%,这主要是由于反应溶液中的 浓度会随着NH4HCO3用量的提高而上升;NH4HCO3浓度继续增大,脱氯率稳定在98%左右不再增加。
浓度会随着NH4HCO3用量的提高而上升;NH4HCO3浓度继续增大,脱氯率稳定在98%左右不再增加。
图5所示为不同NH4HCO3浓度下转化产物的XRD谱。由图5可知,当NH4HCO3浓度小于1.44 mol/L时,反应180 min后,产物主要是Pb3(CO3)2(OH)2和PbCO3的混合物;随着NH4HCO3浓度的不断提高,超过1.56 mol/L时,由于反应溶液中 的浓度升高,
的浓度升高, 在溶液中的扩散加快,可以与PbCl2粒子更好的接触并发生转化反应,产物最终以PbCO3为主。
在溶液中的扩散加快,可以与PbCl2粒子更好的接触并发生转化反应,产物最终以PbCO3为主。
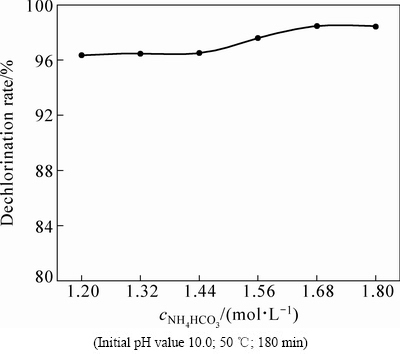
图4 NH4HCO3浓度对脱氯率的影响
Fig. 4 Effect of NH4HCO3 concentration on dechlorination rate

图5 不同NH4HCO3浓度下产物XRD谱
Fig. 5 XRD patterns of products at different NH4HCO3 concentrations
2.4 转化时间的影响
固定其他的反应条件,通过改变反应时间,考察其对氯化铅物相转化过程的影响。结果表明,反应时间对PbCl2脱氯率有很大影响,如图6所示。由图6可看出,PbCl2从开始逐渐反应到30 min时,脱氯率迅速攀升至84.72%;反应时间继续延长至120 min时,脱氯率由84.72%增加至99.47%,但此后继续延长反应时间对脱氯率影响不大,基本恒定在99.50%左右,说明120 min后PbCl2的转化已十分彻底。
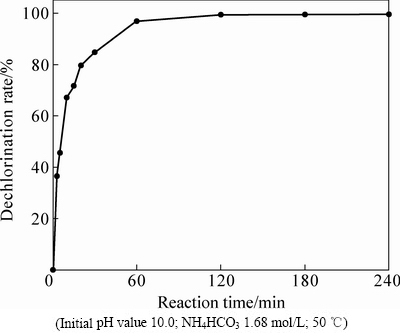
图6 反应时间对脱氯率的影响
Fig. 6 Effects of reaction time on dechlorination rate
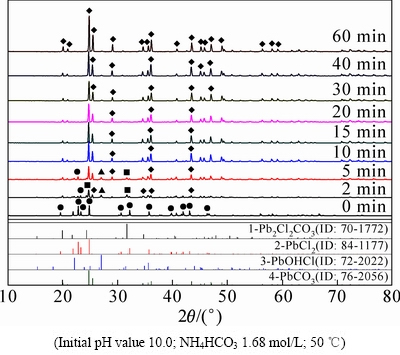
图7 不同转化时间下产物XRD谱
Fig. 7 XRD patterns of products at different reaction time
图7所示为不同反应时间下转化产物的XRD谱。由图7可知,随着脱氯转化反应时间的延长,转化产物的物相组成也在不断发生改变。在反应3 min后,产物中便有大量Pb2Cl2(CO3)和Pb2CO3生成,还有少量PbOHCl,当反应进行10 min以后,产物的物相逐渐由PbOHCl和Pb2Cl2(CO3)转变为Pb2CO3,并趋于稳定,最后以纯净的Pb2CO3为主,反应时间的延长不会再对产物组成造成影响。
2.5 温度的影响
固定其他的反应条件,通过改变反应温度,考察其对氯化铅物相转化过程的影响,结果如图8所示。由图8可看出,当反应温度由30 ℃提高至50 ℃时,脱氯率由96.74%增加至99.66%,这主要是由于提高温度有助于降低溶液黏度,改善搅拌效果,加速铅离子、氯离子和碳酸根离子的扩散速度;此后,继续升高反应温度对增加脱氯率的作用较小,脱氯率基本保持为98%~99%。
图9所示为不同温度下转化产物的XRD谱。由图9可知,反应温度对转化产物的物相组成影响较小,只有在30 ℃时检测到少量Pb3(CO3)2(OH)2物相,其余研究的温度内,转化产物均为PbCO3。

图8 温度对脱氯率的影响
Fig. 8 Effects of temperature on dechlorination rate

图9 不同温度下产物XRD谱
Fig. 9 XRD patterns of products at different reaction temperatures
3 结论
1) 通过实验研究,得出在同一工艺条件下,转化剂的转化脱氯效果由大到小依次为NH4HCO3、Na2CO3、(NH4)2CO3、K2CO3,选择氨水为pH值调节剂、NH4HCO3作为转化剂时的最佳条件为:初始pH值为10.0、NH4HCO3浓度1.68 mol/L、反应温度50 ℃,反应时间120 min。在最佳工艺条件下到99%以上的PbCl2可被转化为纯净的PbCO3。
2) 通过对不同条件、不同转化阶段得到的产物的物相分析,弄清PbCl2在NH4HCO3溶液中转化为PbCO3的机理、途径及影响机制。PbCl2在转化过程中会经历PbCl2 Pb2Cl2(CO3)
Pb2Cl2(CO3) PbCO3的转化过程,但当pH值小于10.0时,溶液中
PbCO3的转化过程,但当pH值小于10.0时,溶液中 浓度不足,PbCl2也会经PbCl2
浓度不足,PbCl2也会经PbCl2 PbOHCl
PbOHCl PbCO3的转化过程;当pH值大于11.0时,产物PbCO3会经历PbCO3
PbCO3的转化过程;当pH值大于11.0时,产物PbCO3会经历PbCO3 Pb3(CO3)2(OH)2再转化过程,使得转化产物PbCO3纯度降低。
Pb3(CO3)2(OH)2再转化过程,使得转化产物PbCO3纯度降低。
3) 转化体系的pH值是影响转化率、转化途径及最终产物物相组成的最显著因素,而转化剂浓度、反应时间和温度,只会对PbCl2脱氯速率造成影响,并不会影响PbCl2的转化途径和最终产物组成。
REFERENCES
[1] 陈进中, 曹华珍, 郑国渠, 支 波, 杨天足. 高锑低银类铅阳极泥制备五氯化锑新工艺[J]. 中国有色金属学报, 2008, 18(11): 2094-2099.
CHEN Jin-zhong, CAO Hua-zhen, ZHENG Guo-qu, ZHI Bo, YANG Tian-zu. Novel technology for preparation of SbCl5 from lead anode slime with high antimony and low silver content[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(11): 2094-2099.
[2] 祝玲钰, 杨巧玉, 陈进中, 陈 平, 郑国渠. 基于超结构模型的铅阳极泥制备锑白工艺水网络优化[J]. 中国有色金属学报, 2012, 22(2): 592-598.
ZHU Ling-yu, YANG Qiao-yu, CHEN Jin-zhong, CHEN Ping, ZHENG Guo-qu. Water network optimization of Sb recovery from lead anode slime based on superstructure[J]. The Chinese Journal of Nonferrous Metals, 2012, 22(2): 592-598.
[3] 中南矿冶学院冶金研究室. 氯化冶金[M]. 北京: 冶金工业出版社, 1976: 182-203.
Central South Institute of Mining and Metallurgy. Chloridizing metallurgy[M]. Beijing: Metallurgical Industry Press, 1976: 182-203.
[4] 李淑梅. 氯化湿法冶金研究进展[J]. 有色矿冶, 2010, 26(3): 34-37.
LI Shu-mei. A review: The research progress of chloridizing metallurgy[J]. Non-ferrous Mining and Metallurgy, 2010, 26(3): 34-37.
[5] TIAN X, GONG Y, WU Y, AGYEIWAA A, ZUO T. Management of used lead acid battery in China: Secondary lead industry progress, policies and problems[J]. Resources Conservation & Recycling, 2014, 93(12): 75-84.
[6] TIAN X, WU Y, GONG Y, ZUO T. The lead-acid battery industry in China: Outlook for production and recycling[J]. Waste Management & Research, 2015, 33(11): 1-9.
[7] 邱德芬, 柯昌美, 王 茜, 张松山. 从废铅膏中回收铅及铅的化合物的方法[J]. 无机盐工业, 2014, 46(7): 16-19.
QIU De-fen, KE Chang-mei, WANG Qian, ZHANG Song-shan. Methods of recovering lead and lead compounds from spend lead paste[J]. Inorganic Chemicals Industry, 2014, 46(7): 16-19.
[8] MORACHEVSKII A G, VAISGANT Z I, RUSIN A I, KHABACHEV M N. Removal of sulfur from the active mass of lead battery scrap[J]. Russian Journal of Applied Chemistry, 2001, 74(7): 1103-1105.
[9] ARAI K, TOGURI J. Leaching of lead sulphate in sodium carbonate solution[J]. Hydrometallurgy, 1984, 12(1): 49-59.
[10] GONG Y, DUTRIZAC J, CHEN T. The conversion of lead sulphate to lead carbonate in sodium carbonate media[J]. Hydrometallurgy, 1992, 28(3): 399-421.
[11] GONG Y, DUTRIZAC J, CHEN T. The reaction of anglesite (PbSO4) crystals with sodium carbonate solutions[J]. Hydrometallurgy, 1992, 31(3): 175-199.
[12] LU K Y, CHEN C Y. Conversion of galena to lead carbonate in ammonium carbonate solution—A new approach to lead hydrometallurgy[J]. Hydrometallurgy, 1986, 17(1): 73-83.
[13] 陈永明, 薛浩天, 李有刚, 杨声海. 氯化铅在碳酸氢铵溶液中的物相转化工艺研究[J]. 湿法冶金, 2014, 5(33): 398-402.
CHEN Yong-ming, XUE Hao-tian, LI You-gang, YANG Sheng-hai. Phase transformation of lead chloride in ammonium bicarbonate solution[J]. Hydrometallurgy of China, 2014, 5(33): 398-402.
[14] 王淼生. 从氯化铅渣中回收铅铋的研究[J]. 材料研究与应用, 1996, 6(1): 39-44.
WANG Miao-sheng. Study on recovering lead and bismuth from lead chloride slag[J]. Journal of Guangdong Nonferrous Metals, 1996, 6(1): 39-44.
[15] LIN G, YU H, LI J. Process for producing basic lead carbonate[P]. US. 2013, US8568670.
[16] 唐谟堂, 陈进中, 蔡传算. 铅锑精矿氯化浸出渣处理新工艺 (I)——苏打转化研究[J]. 中南工业大学学报, 1996, 27(2): 164-167.
TANG Mo-tang, CHEN Jin-zhong, CAI Chuan-suan. Anew technology for treating the residues from chlorination leaching jamesonite concentrate (I)——Study on conversion with soda[J]. Journal of Central South University of Technology, 1996, 27(2): 164-167.
[17] CHEN J Z, CAO H Z, BO L I, YUAN H J, ZHENG G Q, YANG T Z. Thermodynamic analysis of separating lead and antimony in chloride system[J]. Transaction of Nonferrous Metals Society of China, 2009, 19(3): 730-734.
[18] TSUGITA Y. Problems and prospects of halogen element contained dust treatment in recycling[J]. Materials Transactions, 2003, 44(12): 2422-2426.
[19] WOOSLEY R J, MILLERO F J. Pitzer model for the speciation of lead chloride and carbonate complexes in natural waters[J]. Marine Chemistry, 2013, 149: 1-7.
Phase transformation mechanism of lead chloride in ammonium bicarbonate solution
LI Yun1, CHEN Yong-ming1, XUE Hao-tian2, TANG Chao-bo1, YANG Sheng-hai1, TANG Mo-tang1
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Qinghai Provincial Research and Design Academy of Environmental Sciences, Xining, 810000, China)
Abstract: The phase transformation mechanism of PbCl2 in ammonium bicarbonate solution was investigated. The dechlorination efficiencies of different conversion agent in the same condition were determined as follow from big to little: NH4HCO3, Na2CO3, (NH4)2CO3, K2CO3. The optimum transforming conditions of PbCl2 using NH4HCO3 as transforming agent were also detected and determined as follow: initial pH=10.0, cNH4HCO3=1.68 mol/L, reacting temperature 50 ℃, reaction time 120 min. 99% PbCl2 transforms into PbCO3 through PbCl2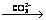 Pb2Cl2(CO3)
Pb2Cl2(CO3) 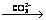 PbCO3 process. The final resultant is pure PbCO3. The phase analysis of conversion products at different conversion stages were carried out to investigate the transformation and influencing mechanism. The results indicate that pH value of the solution is the most significant influencing factor on PbCl2 conversion rate, transformation path and final resultant compositions. While, the NH4HCO3 concentration, reaction time and temperature only affect the dechlorination rate, and don’t affect the transformation path of PbCl2 and the compositions of the conversion products.
PbCO3 process. The final resultant is pure PbCO3. The phase analysis of conversion products at different conversion stages were carried out to investigate the transformation and influencing mechanism. The results indicate that pH value of the solution is the most significant influencing factor on PbCl2 conversion rate, transformation path and final resultant compositions. While, the NH4HCO3 concentration, reaction time and temperature only affect the dechlorination rate, and don’t affect the transformation path of PbCl2 and the compositions of the conversion products.
Key words: lead chloride; ammonium bicarbonate; phase transformation mechanism; dechlorination rate; transformation product
Foundation item: Project (2011AA061001) supported by the National High Technology Research and Development Program of China; Project (2016B020242001) supported by Applied Science and Technology Specialized Research Project of Guangdong Province, China
Received date: 2016-07-15; Accepted date: 2016-11-28
Corresponding author: CHEN Yong-ming, Tel: +86-18684685548; E-mail: csuchenyongming@163.com
(编辑 李艳红)
基金项目:国家高技术研究发展计划资助项目(2011AA061001);广东省应用型科技研发专项资助(2016B020242001)
收稿日期:2016-07-15;修订日期:2016-11-28
通信作者:陈永明,副教授,博士;电话:18684685548;E-mail:csuchenyongming@163.com