文章编号:1004-0609(2016)-12-2686-08
湿法回收废铅膏中间产物柠檬酸铅Pb3(C6H5O7)2·3H2O的热分解
朱新锋1,王现丽1,聂鹏茹2,张 伟2,胡雨辰2,杨家宽2,汪 潇1,郭一飞1
(1. 河南城建学院 市政与环境工程学院,平顶山 467036;
2. 华中科技大学 环境科学与工程学院,武汉 430074)
摘 要:借助热天平与红外光谱仪联用仪、X射线衍射仪、扫描电镜分析手段研究柠檬酸铅Pb3(C6H5O7)2·3H2O的热分解过程和产物。结果表明:柠檬酸铅Pb3(C6H5O7)2·3H2O的热分解过程分水分脱除、无水物分解和燃烧3个阶段;结晶水在无水物分解阶段散失,温度达到100~200 ℃时产生部分有机物,超过此温度时产物主要是CO2,最终产物为氧化铅与金属铅;柠檬酸铅在空气中分解受焙烧温度影响较为显著,柠檬酸铅前驱物Pb3(C6H5O7)2·3H2O低温焙烧时产物的主要成分是α-PbO、β-PbO与金属铅,高温焙烧时产物主要成分是β-PbO。
关键词:柠檬酸铅;热分解;机理;铅膏中间产物
中图分类号:TG156 文献标志码:A
铅酸蓄电池由于其价格相对较为低廉、运行使用时可靠性强、能够承受大电流放电及较宽的适宜环境温度范围等优点而在化学电源中一直占有绝对优势。铅酸蓄电池是世界上各类电池中产量最大、在交通、通信、电力、军事等领域使用最广泛。由于铅酸蓄电池其使用寿命只有2~4年,因超过使用期限而报废的铅酸蓄电池数量正在不断增长,对废旧铅酸蓄电池的回收尤为必要。废铅酸蓄电池的主要组成部分有4个[1]:电解液、板栅、铅膏以及有机物。电池充放电过程中会造成浆状物质的积累,这种浆状物质就是铅膏,约占电池总量30%~40%。铅膏的主要成分是大量的硫酸盐和不同价态的铅的氧化物,其中成分的复杂性是铅酸电池回用困难的主要原因。通用的火法冶炼过程会产生SO2和铅尘等污染物,同时伴随着高耗能和低回收率的弊端[2-3]。因此,本文作者所在团队研究利用废铅膏湿法浸出低温焙烧制备超细铅粉新工艺[4-10]。新工艺有两个关键步骤:1) 快速高效地获得柠檬酸铅;2) 柠檬酸铅能顺利分解制备符合工业应用的铅粉。前期研究发现,柠檬酸-柠檬酸钠体系中得到的是板状柠檬酸铅,分子式为Pb(C6H6O7)·H2O,焙烧温度及延长焙烧时间会对产物组成、晶形及形貌有较大的影响[6, 9]。为了提高反应速度,开发乙酸-柠檬酸钠体系下湿法浸出低温焙烧制备超细铅粉工艺,乙酸-柠檬酸钠体系中制备得到的柠檬酸铅是柱状结构,分子式为Pb3(C6H5O7)2·3H2O,与此前得到的板状柠檬酸铅产物有较大的区别[11]。为了确定柠檬酸铅晶体的分子结构与形貌对焙烧产物的影响,本文作者针对柱状结构柠檬酸铅Pb3(C6H5O7)2·3H2O的热分解过程及制备超细铅粉规律进行研究。
1 实验
1.1 原料及试剂
实验原料为实际铅膏,组分见表1。溶液体系为乙酸-柠檬酸钠体系,柠檬酸钠与铅的摩尔比为4:3,溶液pH为5~6。试验用铅膏起始质量为10 g,按照比例计算可知柠檬酸钠质量为14.2 g,乙酸与铅的摩尔比为8:3,可知乙酸质量为为5.8 g,双氧水与二氧化铅摩尔比为2:1,固液比为1:5。先配制乙酸-柠檬酸钠溶液,加入铅膏,再缓慢加入双氧水,室温下反应2 h。采用两种后处理方式得到柠檬酸铅样品,一种方法是混合液直接过滤、洗涤,烘干得到直接过滤的柠檬酸铅。另外一种方法是反应后的混合液在55 ℃水浴条件下陈化结晶4 h后,进行过滤,洗涤、烘干得到结晶柠檬酸铅。对得到样品采用SEM、XRD、TG/DTA等表征。没有特殊说明指的均为结晶的柠檬酸铅样品。
在不同温度下对柠檬酸铅前驱体进行焙烧,焙烧温度为200、250、300、350、400、450和500 ℃,单次焙烧量5.0 g,焙烧时间1 h。采用XRD、SEM/EDX等手段对焙烧产物进行表征,研究柠檬酸铅热分解机理。
表1 铅膏样品的组分
Table 1 Chemical compositions of lead paste sample (mass fraction, %)

控制Diamond TG/DTA热分析仪的温度为范围25~800 ℃,升温速率5 ℃/min对样品进行热质量损失分析。XRD与FSEM表征分别采用荷兰的X’Pert PRO型X射线衍射仪和Sirion 200,XRD实验条件:铜靶材,Ni滤波,管压40 kV,管流40 mA。表面观测及能谱分析采用SEM/EDS检测仪。柠檬酸铅的热分解过程中质量和红外光谱的变化观测采用型号为Pyris1TGA热天平与红外光谱仪联用仪(TG-FTIR)。将分解气体产物通入红外室进行红外扫描,保证连通设备不漏气,扫描的IR波数范围500~4000 cm-1,分辨率2.5 cm-1,扫描速率每5 s/次,载气流量0.12 L/min。气室及管路温度200 ℃,避免挥发性及半挥发性气体产物产生冷凝和吸收的反应。
1.2 柠檬酸铅的组成
Pb2+的半径为0.119~0.149 nm,且配位数较高,容易与柠檬酸形成柠檬酸铅Pb(C6H6O7)·H2O[12]和Pb6(H2O)2(C6H5O7)4·3H2O[13]等。因此,该实验条件下生成的柠檬酸铅详细元素组分对于后续超细铅粉的热分解制备十分重要。直接过滤的柠檬酸铅、55 ℃后陈化后得到前驱体柠檬酸铅及外购的柠檬酸铅XRD谱如图1所示。
由图1可知,这3种柠檬酸铅的XRD谱具有较大的差异,浸出完成后,铅膏的XRD谱中没有PbSO4、PbO、PbO2的衍射峰,证实了浸出反应完全。结晶产物柠檬酸铅XRD和外购的柠檬酸铅(阿法埃莎公司生产)柠檬酸铅Pb3(C6H5O7)2·3H2O的XRD谱具有较高的相似度。而直接过滤的柠檬酸铅与前两者相比表现出较大的差异性。与SONMEZ等[14]研究硫酸铅在柠檬酸-柠檬酸钠得到柠檬酸铅XRD谱一致。

图1 不同处理方式得到的柠檬酸铅的XRD谱
Fig. 1 XRD patterns of different lead citrate filtering immediately after leaching(a), recrystallization (55℃, 4 h)(b), lead citrate purchased from Alfa Aesar China (Tianjin) Co., Ltd(c)
柠檬酸铅的C、H元素分析显示:C和H元素的质量比分别为6.82与1.49。而分子组成为Pb3(C6H5O7)2·3H2O的柠檬酸铅C、H元素的质量比为6.837与1.519。这两组数据具有较高的吻合度,相对误差小于2 %。鉴于以上数据和XRD结果可确定产物柠檬酸铅的分子式为Pb3(C6H5O7)2·3H2O。溶解产物柠檬酸铅,测定铅含量,结果显示柠檬酸铅的达到了99.9%。图2所示为柠檬酸铅Pb3(C6H5O7)2·3H2O的SEM像。由图2可知,柠檬酸铅呈规则的长柱状,长度为10~50 μm,截面面积约为4 μm2。
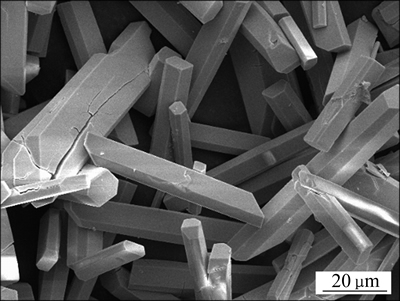
图2 产物柠檬酸铅的SEM像
Fig. 2 SEM image of lead citrate Pb3(C6H5O7)2·3H2O
2 结果与分析
2.1 柠檬酸铅的质量损失特性曲线分析
在空气气氛中对产物柠檬酸铅Pb3(C6H5O7)2·3H2O进行TG-DTA分析,其结果如图3所示。由图3可知,柠檬酸铅在空气中的热分解明显可分为3个阶段:第一阶段在200 ℃以下,为失水阶段,该阶段质量损失约为5.5 %,略大于结晶水含量;第二阶段在200~350 ℃之间,在此区间内300 ℃和335 ℃出DTA出现较为显著的放热峰,这是由于部分柠檬酸铅分解开始氧化放热,此阶段质量损失在31%左右;第三阶段温度在350 ℃以上,在345 ℃时出现强放热峰,该放热峰属于铅燃烧放热,而在415 ℃下的放热峰为炭燃烧放热,该阶段质量损失约4.0%。到450 ℃及以上分解达到稳定状态,总质量损失约为39.6%。
2.2 柠檬酸铅热分解过程分析
在不同的焙烧温度下产生的气相FTIR谱如图4所示。相应的气体红外特征峰吸收波数如表1所示。结合表1与图4可知,焙烧温度在230 ℃气相成分主要为CO2,温度达到330 ℃时,CO2的含量逐渐上升,产生的气体中CO2占90%以上,随温度的升高CO2产气量逐渐减少,温度达到450 ℃时,不再有气体产生。这一现象符合柠檬酸铅的TG-DTA中热分解过程特征。温度高于200 ℃时,在波数为1700~1900 cm-1的范围内出现一些吸收峰,它们可能是乙醛(CH3CHO)、甲醛(CH2O)、甲醇(CH3OH)和甲酸(HCOOH)等[15-16]。从热分解的温度来看,柠檬酸铅Pb3(C6H5O7)2·3H2O更容易分解。与先前研究结果文献[9]中的中柠檬酸铅Pb(C6H6O7)2·H2O的热分解结果相比较,柠檬酸铅Pb3(C6H5O7)2·3H2O分解产生气体明显少于柠檬酸铅Pb(C6H6O7)2·H2O热分解产生气体,从热分解的温度来看,柠檬酸铅Pb3(C6H5O7)2·3H2O的分解温度更低。
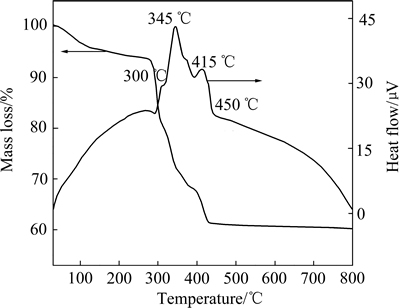
图3 柠檬酸铅的TG-DTA曲线
Fig. 3 TG-DTA curves of lead citrate

图4 柠檬酸铅不同温度热分解的FTIR谱
Fig. 4 FTIR spectra of lead citrate at different temperatures in air
表1 典型气体的特征峰波数
Table 1 Absorption peaks of typical gases

2.3 利用柠檬酸铅焙烧制备超细铅粉
2.3.1 温度对柠檬酸铅烧失量的影响
不同的温度下柠檬酸铅的烧失量如图5所示。由图5可知,在200~250 ℃之间,柠檬酸铅的烧失量循迅速上升;250 ℃后,柠檬酸铅的烧失量上升趋于平缓;而超过350 ℃后,烧失量基本不再变化。主要原因是250 ℃之前,柠檬酸铅发生的反应是脱水以及柠檬酸铅的热分解,在这个过程质量损失的主要原因是结晶水以及柠檬酸铅分解为有机气体以及二氧化碳,造成烧失量快速增加。反应后期主要是剩余碳的燃烧,因此,反应后期质量损失变化不大。
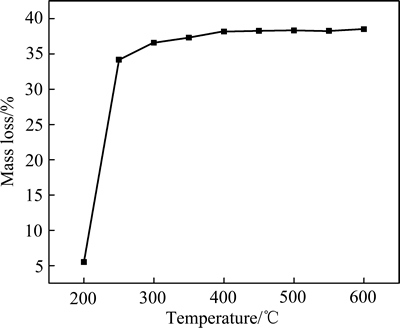
图5 不同温度下柠檬酸铅的质量损失曲线
Fig. 5 Mass loss curves of lead citrate at different temperatures
2.3.2 不同温度下的焙烧产物XRD谱
不同焙烧温度以及不同焙烧时间的条件下制备得到铅粉样品的XRD谱如图6所示。由图6(a)可知,物相变化与焙烧温度密切相关。在300~350 ℃时,焙烧产物包括α-PbO、β-PbO与金属铅。随着焙烧温度的升高,金属铅的含量逐渐变低,而β-PbO的含量逐渐增多,500 ℃下恒温1 h的产物已经完全是β-PbO,这与文献[17]中报道一致。由以上分析可知,随温度升高,氧化量逐渐上升,Pb含量逐渐减小,调节温度可控制Pb的氧化程度及PbO的组成。

图6 不同焙烧温度不同焙烧时间下产物的XRD谱
Fig. 6 XRD patterns of combustion-synthesis products at different temperatures and different time
控制焙烧时间为3 h对不同温度下焙烧的柠檬酸铅样品进行XRD分析,实验结果见图6(b)。由图6(b)可知,不同焙烧温度下的样品的物相有较大的差异。焙烧温度在300~350 ℃时,产物包括α-PbO、β-PbO与金属铅。随着焙烧温度的升高,α-PbO向β-PbO转化,在500 ℃温度下恒温3 h的样品完全转化为β-PbO。恒温1 h的产物与恒温3 h的产物相似,但焙烧3 h的产物比焙烧1 h时的产物中β-PbO增多。柠檬酸铅Pb3(C6H5O7)2·3H2O不同焙烧温度下恒温6 h的样品进行XRD分析,实验结果见图6(c)。由图6(c)可知,XRD分析结果与焙烧3 h的铅粉结果几乎没有差异,没有出现Pb3O4。这与之前的研究结果[9]不同,这可能与柠檬酸铅分解中所能提供的与Pb结合的氧原子数量的差异有关。
2.3.3 不同温度下焙烧产物的SEM像分析
柠檬酸铅Pb3(C6H5O7)2.3H2O不同的温度下焙烧的铅粉的SEM像见图7。由图7可知,焙烧温度为300 ℃时,颗粒由平滑柱状转化为多孔柱状,颗粒粒径有所增加,这是焙烧过程中气体溢出造成的;到350 ℃时,多孔柱状物质更明显;450 ℃时,产物中出现了大量的球状物质,粒径约为20 mm。
柠檬酸铅Pb3(C6H5O7)2·3H2O在350 ℃下恒温不同时间得到焙烧产物见图8。由图8可看出,随着焙烧时间的延长,团聚状态加强,多孔的条状结构逐渐向球形颗粒转换,球形微粒含量上升,多孔的条状结构逐渐向球形颗粒转换的机制可能是在反应过程放出大量的气体,形成条状多孔结构。同时,焙烧温度升高后,球形颗粒增加,说明团聚现象增强,这个过程中促进了条状结构向球形团聚结构的转化。因此,可初步判断柠檬酸铅Pb3(C6H5O7)2·3H2O 在350 ℃下恒温1 h是合适的制备铅粉的焙烧条件。
2.4 柠檬酸铅热分解的机理

图7 不同焙烧温度焙烧1 h制备铅粉的SEM像
Fig. 7 SEM images of combustion-synthesis products at different temperatures for 1 h
根据柠檬酸铅分子式为Pb3(C6H5O7)2·3H2O,组成只有铅、氧、碳3种元素,因此,最终的热分解产物只可能包括铅、氧、碳3种元素。从热分解方程式来分析:由于空气处于过量供应的状态,柠檬酸铅Pb3(C6H5O7)2·3H2O氧化完全,固态产物为PbO,方程式如式(1)所示:
Pb3(C6H5O7)2·3H2O+9O2→3PbO+12CO2+8H2O (1)
在此情况下固体产物为PbO,柠檬酸铅Pb3(C6H5O7)2.3H2O质量损失率为36.4%。
假设氧气供应充足,分解固态产物以金属Pb为主,其方程式如式(2)所示:
Pb3(C6H5O7)2·3H2O+7.5O2→3PbO+12CO2+8H2O (2)
在此情况下,固态产物为Pb和Pb3(C6H5O7)2.3H2O的质量损失达到41.0%。
但是这是两种极限情况的理论计算,在实际过程中,氧气的因素至关重要的,由于在反应过程中还有产生碳,需要氧气存在保证碳的充分燃烧,以防止空气不足,碳将PbO还原为金属铅,降低PbO和铅的摩尔比。可能会发生主要反应见方程式(3)~(5):
C+O2→CO2 (3)
2Pb+O2→2α-PbO (4)
2α-PbO+C→2Pb+CO2 (5)
Pb3(C6H5O7)2.3H2O前驱物在空气中热分解方程式可表示为式(6)。式中x和y值分别代表焙烧产物中PbO和Pb的含量比例,可通过改变焙烧条件(焙烧温度、焙烧时间)来实现不同值。
 →
→ (6)
(6)
从实验结果看,质量损失稳定在38.0%。可以认为PbO和铅组成了产物的主要部分。EDS表征显示:350 ℃焙烧1 h产物的元素组成只有铅、氧,因此,可以认为产物化学成分为PbO和铅。由以上推理可知:柠檬酸铅的热分解过程主要包括200 ℃内的结晶水散失;200~450 ℃内柠檬酸铅分解产生CO2、CO等产物;450 ℃以上的氧化燃烧最终产物为铅与氧化铅。
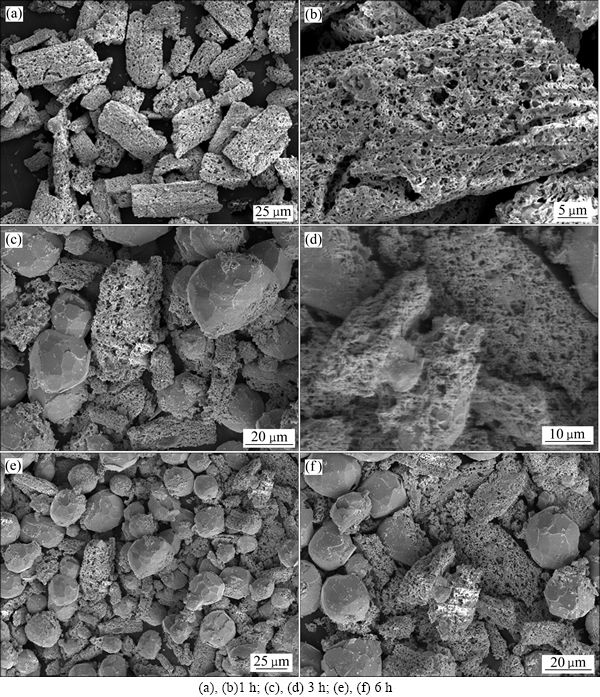
图8 经350 ℃焙烧不同时间后超细粉的SEM像
Fig. 8 SEM images of combustion products of precursor at 350 ℃
3 结论
1) 柠檬酸铅在空气气氛中的热分解过程由脱水、无水物热分解及炭化氧化阶段。柠檬酸铅初始热分解阶段为结晶水散失,在100~200 ℃范围内,部分有机物析出,而此后主要产物为二氧化碳。
2) 柠檬酸铅烧失量随温度升高而,350 ℃以上时,柠檬酸铅烧失量稳定,基本分解完全,其热分解产物是α-PbO、β-PbO与铅。焙烧温度升高,铅氧化为PbO,α-PbO向β-PbO转化,500 ℃温度下恒温1 h产物完全转化为β-PbO。
3) 随焙烧温度提高与焙烧时间延长,焙烧产物晶相向β-PbO转化,多孔板状结构变成球形转化。最适宜焙烧温度为350 ℃,焙烧时间为1 h。
REFERENCES
[1] FERRACIN L C,  A E, DAYOGLIO R A, ROCHA L O, CAFFEU D J, FONTANETTI A R, ROCHA-FILHO R C, BIAGGIO S R, BOCCHI N. Lead recovery from a typical Brazilian sludge of exhausted lead-acid batteries using an electrohydrometallurgical process[J]. Hydrometallurgy, 2002, 65(2/3): 137-144.
A E, DAYOGLIO R A, ROCHA L O, CAFFEU D J, FONTANETTI A R, ROCHA-FILHO R C, BIAGGIO S R, BOCCHI N. Lead recovery from a typical Brazilian sludge of exhausted lead-acid batteries using an electrohydrometallurgical process[J]. Hydrometallurgy, 2002, 65(2/3): 137-144.
[2] VAYSGANT Z, MORACHEYSKY A, DEMIDOY A. A low-temperature technique for recycling lead-acid battery scrap without wastes and with improved environmental control[J]. Journal of Power Sources, 1995, 53(2): 303-306.
[3] COLE J, ERNEST R, LEE A Y. Update on recovering lead from scrap batteries[J]. Journal of Metals, 1985, 37(2): 79-83.
[4] YANG J K, ZHU X F, KUMAR R V. Ethylene glycol-mediated synthesis of PbO nanocrystal from PbSO4: A major component of lead paste in spent lead acid battery[J]. Materials Chemistry and Physics, 2011, 131(1/2): 336-342.
[5] ZHU X F, LI L, SUN X J, YANG D N, GAO L X, LIU J W, KUMAR R V, YANG J K. Preparation of basic leady oxide from spent lead acid battery paste via chemical conversion[J]. Hydrometallurgy, 2012, 117/118: 24-31.
[6] LI L, ZHU X F, YANG D N, GAO L X, LIU J W, KUMAR R V, YANG J K. Preparation and characterization of nano-structured lead oxide from spent lead acid battery paste[J]. Journal of Hazardous Materials, 2012, 203/204(4): 274-282.
[7] LIU J W, YANG D N, GAO L X, ZHU X F, LI L, YANG J K . Effect of iron doped lead oxide on the performance of lead acid batteries[J]. Journal of Power Sources, 2011, 196(20): 8802-8808.
[8] 朱新锋, 杨家宽, 杨丹妮, 孙晓娟, 郭一飞, 陈松涛. 从废铅膏制备超细碳酸铅的表征及热分解性能研究[J]. 功能材料, 2012, 43(27): 2343-2347.
ZHU Xin-feng, YANG Jia-kuan, YANG Dan-ni, SUN Xiao-juan, GUO Yi-fei, CHEN Song-tao. Study on preparation and thermal decomposition of ultra-fine carbonated lead powder[J]. Journal of Functional Materials, 2012, 43(27): 2343-2347.
[9] 朱新锋, 杨家宽, 胡雨辰, 李 磊, 汪 潇, 张 伟, 孙晓娟, 郭一飞, 陈松涛. 柠檬酸铅Pb(C6H6O7)·H2O热分解性能研究[J]. 材料热处理学报, 2014, 35(6): 10-14.
ZHU Xin-feng, YANG Jia-kuan, HU Yu-chen, LI Lei,WANG Xiao, ZHANG Wei, SUN Xiao-juan, GUO Yi-fei, CHEN Song-tao. Study on behavior of thermal decomposition of lead citrate[J]. Transactions of Materials and Heat Treatment, 2014, 35(6): 10-14.
[10] 朱新锋, 刘万超, 杨海玉, 李 磊, 杨家宽. 以废铅酸电池铅膏制备超细氧化铅粉末[J]. 中国有色金属学报, 2010, 20(1): 132-136.
ZHU Xin-feng, LIU Wan-chao, YANG Hai-yu, LI Lei, YANG Jia-kuan. Preparation of ultra-fine PbO powders from lead paste in spent lead acid battery[J]. The Chinese Journal of Nonferrous Metals, 2010, 20(1): 132-136.
[11] ZHU X F, YANG J K, HE X, LI L, GAO L X, YANG D N, SUN X J, ZHANG W, WANG Q, LIU J W, KUMAR R V. Leaching of spent lead acid battery paste components by sodium citrate and acetic acid[J]. Journal of Hazardous Materials, 2013, 250/251(8): 387-396.
[12] KOURGIANTAKIS M, RAPTOPOULOU C P, MATAZPETAKIS M. Lead-citrate chemistry. synthesis, spectroscopic and structural[J]. Inorganica Chimica Acta, 2000, 297(1/2): 134-138.
[13] 石 晶, 徐家宁, 张 萍. [Pb6(H2O)2(cit)4]·3H2O和Pb(tar)- (H2O)2两种柔性酸和铅的配位聚合物的水热合成与表征[J]. 高等学校化学学报, 2007, 28(9): 1617-1621.
SHI Jing, XU Jia-ning, ZHANG Ping. Hydrothermal synthesis and characterization of two coordination polymers of flexible acid and lead: [Pb6(H2O)2(cit)4]·3H2O and Pb(tar)(H2O)2[J]. Chemical Research in Chinese Universities, 2007, 28(9): 1617-1621.
[14] SONMEZ M S, KUMAR R V. Leaching of waste battery paste components. Part 2: Leaching and desulphurisation of PbSO4 by citric acid and sodium citrate solution[J]. Hydrometallurgy, 2009, 95(1): 82-86.
[15] BASSILAKIS R, CARANGELO R M, WOJTOWICZ M A. TG-FTIR analysis of biomass pyrolysis[J]. Fuel, 2001, 80 (12): 1765-1786.
[16] JONG W D, PIRONE A,  M A. Pyrolysis of miscanthus giganteus and wood pellets: TG-FTIR analysis and reaction kinetics[J]. Fuel, 2003, 82(9): 1139-1147.
M A. Pyrolysis of miscanthus giganteus and wood pellets: TG-FTIR analysis and reaction kinetics[J]. Fuel, 2003, 82(9): 1139-1147.
[17] MUNSON M J, RIMAN R E. Observed phase transformations of oxalate-derived lead monoxide powder[J]. Journal of Thermal Analysis, 1991, 37(37): 2555-2566.
Thermal decomposition of lead citrate Pb3(C6H5O7)2·3H2O from recovery spent lead paste by hydrometallurgy process
ZHU Xin-feng1, WANG Xian-li1, NIE Peng-ru2,ZHANG Wei2, HU Yu-chen2, YANG Jia-kuan2, WANG Xiao1, GUO Yi-fei1
(1. College of Municipal and Environmental Engineering, Henan University of Urban Construction, Pingdingshan 467036, China;
2. College of Environmental Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, China)
Abstract: Thermal decomposition behavior and product were systematically investigated by TG-FTIR, XRD, SEM analysis. The results show that the thermal decomposition process of lead citrate is divided into dewatering, organic constituent decomposition and burning stages. The dehydrating crystal water occurs in the initial decomposition followed by the generation of intermediate organic matters at 100-200 ℃. Subsequently, the ultimate gas and solid products from this decomposition process are CO2 and a mixture mainly containing PbO and Pb, respectively. The major products of Pb3(C6H5O7)2·3H2O at relatively low temperature are α-PbO, β-PbO and Pb, whereas, β-PbO is the dominant product at relatively higher temperature.
Key words: lead citrate; thermal decomposition; mechanism; lead paste
Foundation item: Project(50804017) supported by the National Natural Science Foundation of China; Project (152102210299) supported by Science and Technology Project of Henan Province Science and Technology Department, China; Project(15A610002) supported by the Key Scientific Research Project of Colleges and Universities in Henan Province, China
Received date: 2015-08-11; Accepted date: 2016-07-28
Corresponding author: YANG Jia-kuan; Tel: +86-27-87792207; E-mail: yjkuan@126.com
(编辑 李艳红)
基金项目:国家自然科学基金资助项目(50804017);河南省科技攻关项目(152102210299);河南省高校重点科研项目(15A610002)
收稿日期:2015-08-11;修订日期:2016-07-28
通信作者:杨家宽,教授,博士;电话:027-87792207;E-mail: yjkuan@126.com