高锰酸钾改性黄芪废渣活性炭对Cd2+的吸附
来源期刊:中国有色金属学报(英文版)2018年第4期
论文作者:冯宁川 范玮 朱美霖 郭学益
文章页码:794 - 801
关键词:黄芪废渣;活性炭;改性;吸附;Cd2+
Key words:Astragalus residue; activated carbon; modification; adsorption; Cd2+
摘 要:以黄芪废渣为原料,用KOH为活化剂制备黄芪废渣活性炭,并用KMnO4改性,改性前后的黄芪废渣活性炭用于对水溶液中Cd2+的吸附。采用扫描电子显微术、比表面积测定、X射线衍射、红外光谱和贝母滴定等方法对改性前后的黄芪废渣活性炭进行表征;通过静态吸附实验考察改性前后黄芪废渣活性炭对水溶液中Cd2+的吸附性能。结果表明,KMnO4改性后活性炭表面含氧官能团增加,MnO2沉积到活性炭表面。改性前后的黄芪废渣活性炭对Cd2+的吸附符合准二级动力学方程,等温吸附更符合Langmuir模型,改性前后的黄芪废渣活性炭对Cd2+的饱和吸附量分别是116.96和217.00 mg/g。KMnO4显著改变了黄芪废渣活性炭的物理化学性质和表面结构,改性后的黄芪废渣活性炭对Cd2+的吸附能力明显提高。
Abstract: Activated carbon obtained from Astragalus residue was chemically activated by KOH and modified with KMnO4. The samples were characterized by N2 adsorption, Fourier transform infrared spectroscopy, X-ray diffractometry, scanning electron microscopy, and Boehm titration. Accordingly, the original and modified carbon materials were used for the removal of Cd2+ from aqueous solution by batch adsorption experiments. Results showed that the contents of oxygen-containing functional groups increased, and MnO2 was nearly uniformly deposited on the surface of activated carbon after modification by KMnO4. The adsorption kinetics was described by pseudo-second order model. Langmuir model fitted the adsorption-isotherm experimental data of Cd2+ better than the Freundlich model. The maximum adsorption capacities of the activated carbon before and after modification for Cd2+ were 116.96 and 217.00 mg/g, respectively. KMnO4 considerably changed the physicochemical properties and surface texture of activated carbon and enhanced the adsorption capacity of activated carbon for Cd2+.
Trans. Nonferrous Met. Soc. China 28(2018) 794-801
Ning-chuan FENG1, Wei FAN1, Mei-lin ZHU1, Xue-yi GUO2
1. School of Basic Medical Science, Ningxia Medical University, Yinchuan 750004, China;
2. School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 19 October 2016; accepted 26 October 2017
Abstract: Activated carbon obtained from Astragalus residue was chemically activated by KOH and modified with KMnO4. The samples were characterized by N2 adsorption, Fourier transform infrared spectroscopy, X-ray diffractometry, scanning electron microscopy, and Boehm titration. Accordingly, the original and modified carbon materials were used for the removal of Cd2+ from aqueous solution by batch adsorption experiments. Results showed that the contents of oxygen-containing functional groups increased, and MnO2 was nearly uniformly deposited on the surface of activated carbon after modification by KMnO4. The adsorption kinetics was described by pseudo-second order model. Langmuir model fitted the adsorption-isotherm experimental data of Cd2+ better than the Freundlich model. The maximum adsorption capacities of the activated carbon before and after modification for Cd2+ were 116.96 and 217.00 mg/g, respectively. KMnO4 considerably changed the physicochemical properties and surface texture of activated carbon and enhanced the adsorption capacity of activated carbon for Cd2+.
Key words: Astragalus residue; activated carbon; modification; adsorption; Cd2+
1 Introduction
Cadmium (Cd2+) is one of the most commonly used heavy metals and widely utilized in industries, such as electroplating, paint pigments, plastics manufacturing, and mining [1]. Cd2+ is toxic even at extremely low concentrations and has been classified as a human carcinogen by the US National Toxicology Program [2]. At present, ion-exchange [3] and chemical precipitation [4] are among the treatments for Cd2+ removal from aqueous media. Additionally, activated carbon adsorption is used for the removal of various heavy metal ions dissolved in aqueous media. In most cases, activated carbon (AC) is synthesized from natural precursors such as coal, wood or nutshells [5,6]. Although these materials are very effective, their widespread use is restricted because of their high costs. Carbon adsorption removes organic compounds but is ineffective on removing metals and inorganic pollutants [7]. In recent years, many researchers have focused on finding economical and available materials for the preparation of activated carbon, especially from plant biomass and biomaterial waste, such as olive stone waste residue [8], and grape bagasse [9]. Activated carbon modification is gaining prominence because of the increasing need to improve the affinity of activated carbon for certain contaminants for their effective removal. Among the current modification techniques, chemical oxidation is the most commonly used owing to its simplicity, low cost, low energy requirement. Additionally, oxygen-containing functional groups can be introduced on activated carbon surfaces through this method. SONG et al [10] used HNO3 and H2O2 as oxidants for modifying activated carbon and investigated the effect of liquid-phase oxidation on the Pb2+ adsorption capacity of activated carbon. Meanwhile, WANG et al [11] applied KMnO4-modified activated carbon for Pb(II) adsorption. The results showed that chemical oxidation can be performed for enhancing the adsorption capacity of activated carbon for small molecules and ions.
Astragalus contains polysaccharides, organic acids, alkaloids, saponins and so on [12], and is commonly used in traditional Chinese medicine. After the main components of this plant are extracted, a large amount of residue, which has no economic value, is generated annually and discarded in the environment, thereby increasing solid waste; in particular, Astragalus residues are disposed in land fillings or incinerated and are thus a source of secondary pollution [13]. Thus, managing Astragalus residue is of great importance. In recent years, Astragalus residue has been used as feed, fertilizer, and substrate of solid-state fermentation. Notably, preparation of activated carbon through the chemical activation of Astragalus residue with KOH has not been reported yet. In the present work, activated carbon was prepared from Astragalus residue, and the obtained activated carbon was modified by KMnO4. The original and modified activated carbons were both applied for the adsorption of Cd2+ in aqueous media.
2 Experimental
2.1 Solution preparation
Double distilled water was used to prepare all solutions, and all reagents were of analytical grade. Stock solutions of Cd2+ at concentrations of 1 g/L were prepared using cadmium nitrate.
2.2 Preparation of activated carbon
Astragalus residue obtained from a local pharmaceutical company in Ningxia, China was used as raw material for the preparation of AC by KOH activation. The residues were pulverized, sieved to particle size (40 mesh), washed with distilled water, and air-dried. Then, 20% (mass fraction) KOH solution was mixed with Astragalus residue at a ratio of 1:3 (residue:KOH, g/mL). The resulting chemical-loaded sample was then heated in a muffle furnace and activated at 600 °C for 80 min. The product was cooled to room temperature, soaked with 0.1 mol/L HCl to remove the residual KOH, and washed sequentially several times with distilled water until its filtrate reached neutral pH. The resulting activated carbon material was dried at 80 °C for 12 h and stored for subsequent use (designed as AC).
2.3 KMnO4 modification of AC
Activated carbon was treated with KMnO4 through dipping and heating methods. Activated carbon was placed into 0.15 mol/L KMnO4 solution at a ratio of 10:1 (activated carbon:KMnO4 solution, g/L) for 90 min at boiling temperature. The modification was conducted under stirring in a flask equipped with a stirrer and a reflux condenser and was heated in a thermostat water bath. Before being dried at 80 °C for 12 h, the modified activated carbon was washed repeatedly with distilled water until the effluent water was neutral. After treatment, the sample was cooled to room temperature and stored in a desiccator for later use (designed as AC-Mn).
2.4 Characterization methods
The surface areas and pore characteristics of the prepared carbons were determined by N2 adsorption/ desorption isotherm at 77 K (Micrometritics, ASAP2020). The surface morphology was observed by scanning electron microscopy (SEM, HITACHI S-3400N). X-ray diffraction (XRD, Shimadzu XRD-6000) patterns were collected by using Cu Kα radiation. The chemical structure of the carbon was qualitatively evaluated with Fourier transform infrared (FTIR) spectrometer (Nicolet, NEXUS670). The Boehm titration method [14] was performed for the qualitative and quantitative identification of the surface functional groups of the activated carbon.
2.5 Batch experiments
Batch adsorption experiments were carried out at 25 °C by agitating 0.060 g of adsorbent with 30 mL Cd2+ solution of desired concentration in 100 mL sealed conical flask. A shaking thermostat machine was used at a speed of 100 r/min for 2 h except for the contact time experiments. The effect of solution pH on the equilibrium adsorption of Cd2+ was investigated by mixing 0.060 g adsorbent with 30 mL of 100 mg/L Cd2+ solution between pH 3.0 and 7.0. Time variations in the range of 2-120 min were used to study the kinetics of the Cd2+ adsorption. In the isotherm experiments, 0.060 g adsorbent was added in 30 mL Cd2+ solution with Cd2+ solution concentration varying from 100 to 800 mg/L at the optimum pH. Once the preset contact time (2 h) was reached, the samples were filtered through a 0.45 μm filter paper. The concentration of Cd2+ in the solution was measured by flame atomic absorption spectrometry (Persee A3 Atomic Absorption Spectrophotometer).
The adsorption capacity, q (mg/g), and removal rate, E (%), of Cd2+ at equilibrium were calculated as follows:
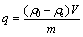 (1)
(1)
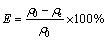 (2)
(2)
where ρ0 and ρe are the initial and equilibrium Cd2+ ion concentrations (mg/L) in the solutions, respectively, V is the volume of solution (L), and m is the mass of adsorbent (g).
3 Results and discussion
3.1 Characterization of adsorbents
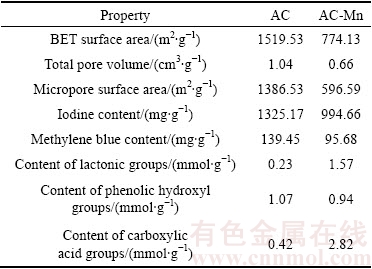
The results of the pore structural characterization of AC and AC-Mn are given in Table 1. AC had a large BET surface area and mostly contained micropores. The presence of micropores enhances the adsorption capacity of AC, especially for small molecules or ions such as Cd2+ [15]. KMnO4 treatment caused the decreases in the BET surface area, total pore volume, and micropore surface area. For the initial AC, the BET surface area was 1519.53 m2/g, which decreased to 774.13 m2/g after modification. Total pore volume and micropore surface area changed significantly. The results indicated that the pore structure of the AC-Mn was destroyed by KMnO4 through strong oxidation at a high temperature.
The physical properties of samples are summarized in Table 1. The amount of iodine (1325.17 mg/g) and that of methylene blue (139.45 mg/g) in AC were higher than those of AC-Mn (994.66 mg/g and 95.68 mg/g, respectively). The iodine number was a measure of micropore content of the activated carbon by adsorption of iodine from solutions [16]. In the present work, the iodine contents of AC and AC-Mn indicated that they are micropore-type activated carbon and thus suitable for the adsorption of small molecules and ions such as Cd2+. This finding corresponded to the result of micropore surface area. The Boehm titration results of AC and AC-Mn are given in Table 1. The number of oxygen- containing functional groups, such as carboxylic and lactone groups, increased after the activated carbon was subjected to oxidation modification by KMnO4. The interactions between the oxygen-containing functional groups and heavy metal ions are strong, indicating that the absorption of heavy metals by activated carbon occurs at its oxygen-containing functional groups [17]. Thus, the functional groups facilitate the adsorption of heavy metal ions.
Table 1 Some properties of activated carbons
The SEM images of AC and AC-Mn are shown in Fig. 1. The surface morphology of AC was different from that of AC-Mn. The surface of AC showed a honeycomb-like and well-developed porous structure. After AC was treated with KMnO4, its surface morphology was completely changed. Conversely, the grainy structure reflected on AC-Mn in Fig. 1(b) revealed that MnO2 was nearly uniformly deposited on the surface because of redox reactions facilitated by the impregnation of KMnO4.
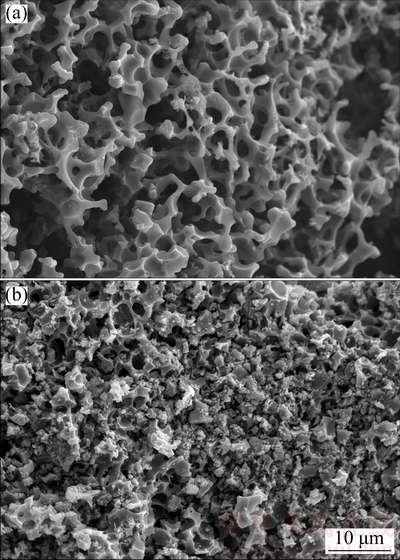
Fig. 1 SEM images of AC (a) and AC-Mn (b)
The XRD spectra of AC and AC-Mn are presented in Fig. 2. The diffraction spectrum of AC (Fig. 2(a)) displayed two diffraction peaks around 26° and 43°, corresponding to the 002 and 100 diffraction planes of graphite [18,19]. The 002 plane presented a narrow peak with steep rise, and the 100 plane displayed a broad small diffraction peak. These characteristics indicated that AC had a predominantly amorphous carbon structure. The diffraction spectrum of AC-Mn is shown in Fig. 2(b). The 002 plane showed peaks with gradually decreased intensity, whereas the 100 plane disappeared. Both results indicated that the aligned structural domains in the carbon matrix had been destroyed in the process of oxidation–reduction. Meanwhile, the extent of graphitization was decreased [19]. However, for the AC-Mn samples, the diffraction peaks at 25.91, 37.13, 42.57 and 65.95 showed the presence of MnO2 [11,20]. These results confirmed that MnO2 was successfully formed as a result of the redox reactions.
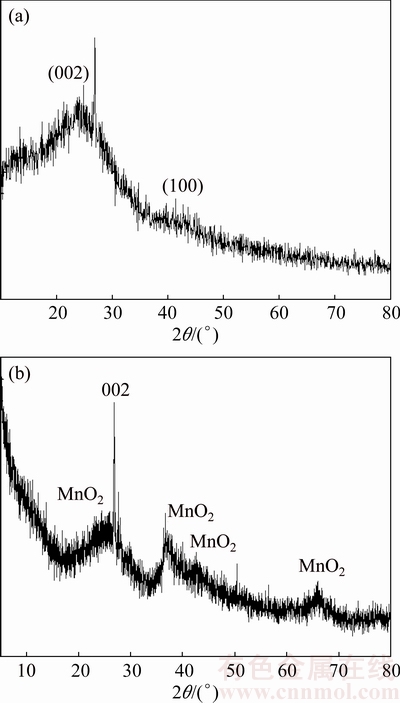
Fig. 2 XRD patterns of AC (a) and AC-Mn (b)
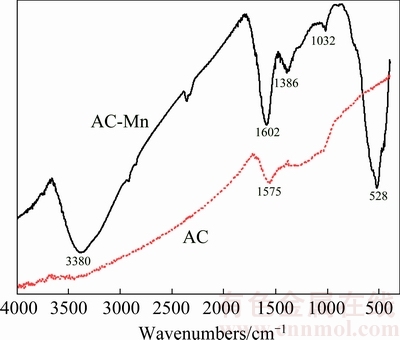
Fig. 3 FTIR spectra of AC and AC-Mn
Figure 3 shows the FTIR spectra of AC and AC-Mn. The FTIR spectrum of AC plotted a band at 1575 cm-1 which was attributed to the double bond (C=C) vibrations in an aromatic system and the highly conjugated C—O stretching vibration bands [21]. Compared with the FTIR spectrum of AC, liquid-phase oxidation modified and produced new IR adsorption peaks. The FTIR spectra of AC-Mn at about 3380, 1386 and 1032 cm-1 can be assigned to the —OH stretching vibration, C—C stretch in ring and C—O stretching vibration [19,22], respectively. The peak at 1602 cm-1 was the stretching vibrations of COO-. An obvious change in the spectrum for AC-Mn was the appearance of a new band at 528 cm-1, and these characteristic peaks were ascribed to the Mn—O vibrations [20]. These results suggested that the oxygen-containing functional groups of AC-Mn increased and also confirmed the presence of MnO2.
Based on this analysis, a simulation scheme was proposed for the illustration of the whole process of AC modification (Scheme 1).
3.2 Results of adsorption experiments
3.2.1 Effect of pH
The influence of the initial pH on the adsorption of Cd2+ ions onto AC and AC-Mn is shown in Fig. 4. The removal rate increased with the increase of pH from 3.0 to 4.0, and then the increase slowed down in pH range of 4.0-7.0. The influence of the pH of the solution on the Cd2+ ion uptake may be attributed to the fact that at lower pH value, the surfaces of AC and AC-Mn were surrounded by H+ ions, which prevented Cd2+ ions from approaching the binding sites of the adsorbents. The electric repulsion of protons resulted in the suppression of Cd2+ adsorption on the adsorbent surface. With increasing pH, the competition from the hydrogen ions decreased and the number of exchangeable binding sites on the surface of adsorbents increased, resulting in the increase in Cd2+adsorption [11,13]. To increase the adsorption capacity and retain the simple form of Cd2+ in the solutions, the pH value of 6.0 was selected for the subsequent batch experiments.
3.2.2 Adsorption kinetics
The removal rates of Cd2+ adsorbed on AC and AC-Mn at different time are presented in Fig. 5. The removal rate of AC and AC-Mn for Cd2+ increased rapidly in the initial stages of contact time and reached equilibrium at 20 min. Afterwards, the removal rate elevated slowly and reached adsorption equilibrium gradually.
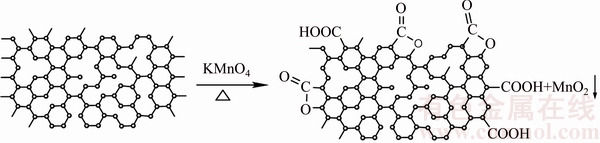
Scheme 1 Schematic for modification of AC
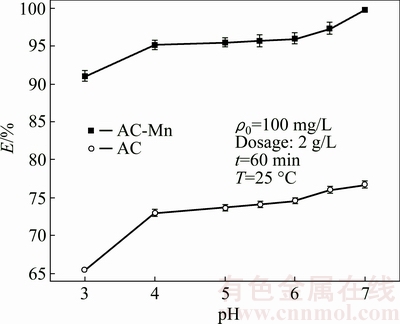
Fig. 4 Effects of pH on removal rate of Cd2+ on AC and AC-Mn
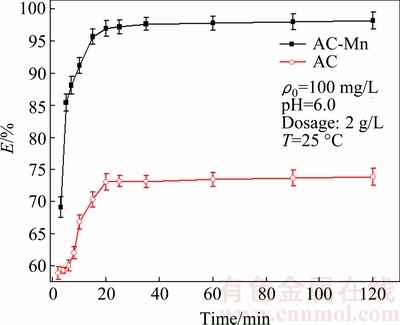
Fig. 5 Effects of contact time on removal rate of Cd2+ on AC and AC-Mn
In order to investigate adsorption kinetics of Cd2+ on AC and AC-Mn, both pseudo-first order and pseudo-second order kinetic models were used to test the experimental data. The pseudo-first order equation is represented in the form [23]
ln(qe-qt)=ln qe-k1t (3)
where k1 is the rate constant of pseudo-first order adsorption, and qe and qt denote the amount of adsorption at equilibrium and at time t, respectively.
The pseudo-second order equation is expressed as [24]
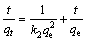 (4)
(4)
where k2 is the pseudo-second order rate constant. The product 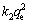 is actually the initial sorption rate represented as h=
is actually the initial sorption rate represented as h=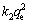 .
.
The kinetic parameters were calculated using Eqs. (3) and (4) and are provided in Table 2. The initial adsorption rate (h) of AC-Mn was significantly larger than that of AC, indicating that the removal rate of the former was faster than that of the latter. The correlation coefficient (R2) for pseudo-second order kinetic model was much closer to 1 than that of the first-order kinetic model, and the adsorption capacities calculated by the second-order model were closer to the experimental results. Therefore, the adsorption of Cd2+ on AC and AC-Mn was effectively described by the pseudo-second order kinetic model, and successful fitting of the pseudo-second order model indicated that chemical adsorption is the rate-controlling step [25].
3.2.3 Adsorption isotherms
The adsorption isotherms of Cd2+ on AC and AC-Mn are shown in Fig. 6. Compared with AC, AC-Mn had higher adsorption capacity for Cd2+ because of its more numerous oxygen-containing functional groups, and the generation of MnO2 increased the adsorption capacity of AC-Mn. MnO2 has a high affinity for many heavy metals, which can be adsorbed as an inner sphere complex by means of surface complexation on MnO2 [26]. In the last decade, using MnO2 as adsorbent for the removal of various heavy metals ions has been attracting attention [26-28].
The experimental data of equilibrium isotherm for Cd2+ on AC and AC-Mn were analyzed with Freundlich (Eq. (5)) [29] and Langmuir (Eq. (6)) isotherms [30]:
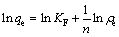 (5)
(5)
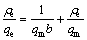 (6)
(6)
where ρe (mg/L) is the equilibrium concentration of Cd2+ ions, qe (mg/g) is the amount of Cd2+ ions adsorbed at equilibrium, qm (mg/g) is the maximum adsorption capacity of Cd2+ ions, b (L/mg) is the Langmuir isotherm coefficient, and KF (mg/g) and n are the Freundlich constants.
Table 2 Kinetic parameters for adsorption of Cd2+ on AC and AC-Mn
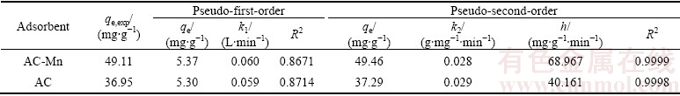
Table 3 Langmuir and Freundlich parameters for adsorption of Cd2+ on AC and AC-Mn
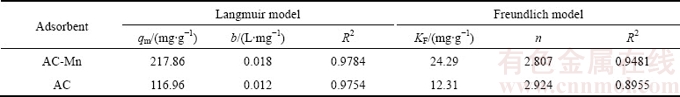
Table 4 Maximum adsorption capacities for Cd2+ by various activated carbons
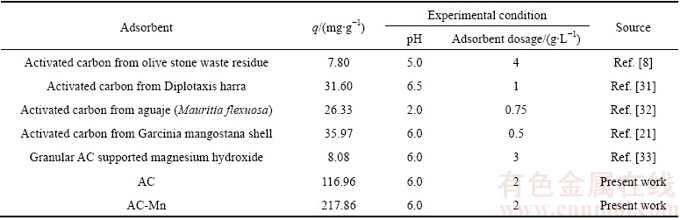
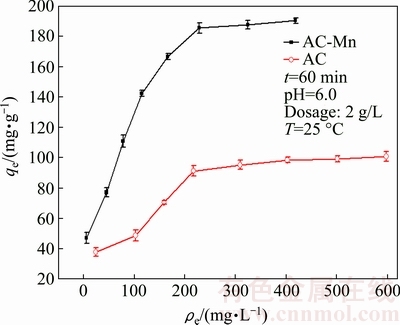
Fig. 6 Adsorption isotherms of Cd2+ on AC and AC-Mn
The parameters of the isotherm models determined from the regression analysis of experimental data are summarized in Table 3. These results indicated that the Langmuir isotherm fitted better than the Freundlich isotherm within the concentration range of research. According to the Langmuir isotherm, the maximum adsorption capacities of AC and AC-Mn for Cd2+ were 116.96 and 217.86 mg/g, respectively, which were much higher than those of AC prepared from other raw materials and commercial AC (Table 4).
4 Conclusions
1) Astragalus residue was used as a precursor for the preparation of AC, and then AC was modified by KMnO4. AC had great BET surface area and porous honeycomb structure. AC-Mn from Astragalus residue loaded a large amount of MnO2, produced more oxygen- containing functional groups on the basis of AC, and had lower BET surface area than AC.
2) AC and AC-Mn were used to adsorb Cd2+ in simulated waste water. The adsorption capacity of AC-Mn for Cd2+ increased and became 1.86 times that of AC because of the increase in its oxygen-containing functional groups and the emergence of MnO2 imparted onto AC by modification. The equilibrium data of Cd2+ on AC and AC-Mn were best fitted to Langmuir isotherm. The adsorption process was found to follow the pseudo-second order kinetic model.
3) AC and AC-Mn were employed as a cheap and effective adsorbent for the removal of Cd2+ from aqueous solutions because of their cost effectiveness and high adsorption capacities.
References
[1] CHAI Li-yuan, CHEN Yun-nen, SHU Yu-de, CHANG Hao, LI Qing-zhu. Adsorption and removal of cadmium(II) from aqueous solutions by bio-formulation [J]. Transactions of Nonferrous Metals Society of China, 2007, 17: 1057-1062.
[2] BIAN Yu, BIAN Zhao-yong, ZHANG Jun-xiao, DING Ai-zhong, LIU Shao-lei, ZHENG Lei, WANG Hui. Adsorption of cadmium ions from aqueous solutions by activated carbon with oxygen-containing functional groups [J]. Chinese Journal of Chemical Engineering, 2015, 23: 1705-1711.
[3] ZEWAIL T M, YOUSRF N S. Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed [J]. Alexandria Engineering Journal, 2015, 54: 83-90.
[4] SIS H, UYSAL T. Removal of heavy metal ions from aqueous medium using Kuluncak (Malatya) vermiculites and effect of precipitation on removal [J]. Applied Clay Science, 2014, 95: 1-8.
[5] LI Peng, AILIJIANG N, CAO Xiao-xin, LEI Ting, LIANG Peng, ZHANG Xiao-yuan, HUANG Xia, TENG Ji-lin. Pretreatment of coal gasification wastewater by adsorption using activated carbons and activated coke [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2015, 482: 177-183.
[6] HEIBATI B, RODRIGUEZ-COUTO S, AL-GHOUTI M A, ASIF M, TYAGI I, AGARWAL S, GUPTA V K. Kinetics and thermodynamics of enhanced adsorption of the dye AR 18 using activated carbons prepared from walnut and poplar woods [J]. Journal of Molecular Liquids, 2015, 208: 99-105.
[7] MONSER L, ADHOUM N. Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater [J]. Separation and Purification Technology, 2002, 26: 137-146.
[8] ALSLAIBI T M, ABUSTAN I, AHMAD M A, FOUL A A. Preparation of activated carbon from olive stone waste: Optimization study on the removal of Cu2+, Cd2+, Ni2+, Pb2+, Fe2+, and Zn2+ from aqueous solution using response surface methodology [J]. Journal of Dispersion Science and Technology, 2014, 35: 913-925.
[9] DEMIRAL H, GUNGOR C. Adsorption of copper(II) from aqueous solutions on activated carbon prepared from grape bagasse [J]. Journal of Cleaner Production, 2016, 124: 103-113.
[10] SONG Xiao-lan, LIU Hong-yan, CHENG Lei, QU Yi-xin. Surface modification of coconut-based activated carbon by liquid-phase oxidation and its effects on lead ion adsorption [J]. Desalination, 2010, 255: 78-83.
[11] WANG Yin, WANG Xue-jiang, WANG Xin, LIU Mian, YANG Lian-zhen, WU Zhen, XIA Si-qing, ZHAO Jian-fu. Adsorption of Pb(II) in aqueous solutions by bamboo charcoal modified with KMnO4 via microwave irradiation [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2012, 414: 1-8.
[12] ELABD H, WANG Han-ping, SHAHEEN A, YAO Hong, ABBASS A. Feeding Glycyrrhiza glabra (liquorice) and Astragalus membranaceus (AM) alters innate immune and physiological responses in yellow perch (Perca flavescens) [J]. Fish & Shellfish Immunology, 2016, 54: 374-384.
[13] MA Chun-fang, FAN Wei, ZHANG Jin-bao, SHI Yang, FENG Ning-chuan. Study of Pb2+, Cd2+ and Ni2+ adsorption onto activated carbons prepared from glycyrrhiza residue by KOH or H3PO4 activation [J]. Water Science and Technology, 2015, 72: 451-462.
[14] BOEHM H P. Some aspects of the surface chemistry of carbon blacks and other carbons [J]. Carbon, 1994, 32: 759-769.
[15] AN Feng-hua, CHENG Yuan-ping, WU Dong-mei, WANG Liang. The effect of small micropores on methane adsorption of coals from Northern China [J]. Adsorption, 2013, 19: 83-90.
[16] CEYHAN A A,  , BAYTAR O, SAKA C. Surface and porous characterization of activated carbon prepared from pyrolysis of biomass by two-stage procedure at low activation temperature and it's the adsorption of iodine [J]. Journal of Analytical and Applied Pyrolysis, 2013, 104: 378-383.
, BAYTAR O, SAKA C. Surface and porous characterization of activated carbon prepared from pyrolysis of biomass by two-stage procedure at low activation temperature and it's the adsorption of iodine [J]. Journal of Analytical and Applied Pyrolysis, 2013, 104: 378-383.
[17] VALLE-VIGON P, SEVILLA M, FUERTES A B. Carboxyl- functionalized mesoporous silica–carbon composites as highly efficient adsorbents in liquid phase [J]. Microporous and Mesoporous Materials, 2013, 176: 78-85.
[18] LU An-hui, ZHENG Jing-tang. Study of microstructure of high- surface-area polyacrylonitrile activated carbon fibers [J]. Journal of Colloid and Interface Science, 2001, 236: 369-374.
[19] XIAO Yong, LONG Cao, ZHENG Ming-tao, DONG Han-wu, LEI Bing-fu, ZHANG Hao-ran, LIU Ying-liang. High-capacity porous carbons prepared by KOH activation of activated carbon for supercapacitors [J]. Chinese Chemical Letters, 2014, 25: 865-868.
[20] PAN Ning, LI Long, DING Jie, LI Sheng-ke, WANG Rui-bing, JIN Yong-dong, WANG Xiang-ke, XIA Chuan-qin. Preparation of graphene oxide-manganese dioxide for highly efficient adsorption and separation of Th(IV)/U(VI) [J]. Journal of Hazardous Materials, 2016, 309: 107-115.
[21] KANG Y L, POON M Y, MONASH P, IBRAHIM S, SARAVANAN P. Surface chemistry and adsorption mechanism of cadmium ion on activated carbon derived from Garcinia mangostana shell [J]. Korean Journal of Chemical Engineering, 2013, 30: 1904-1910.
[22] KARMACHARYA M S, GUPTA V K, TYAGI I.; AGARWAL S, JHA V K. Removal of As(III) and As(V) using rubber tire derived activated carbon modified with alumina composite [J]. Journal of Molecular Liquids, 2016, 216: 836-844.
[23] HAMEED B H, AHMAD A A, AZIZ N. Isotherms, kinetics and thermodynamics of acid dye adsorption on activated palm ash [J]. Chemical Engineering Journal, 2007, 133: 195-203.
[24] HO Y S, MCKAY G. Sorption of copper(II) from aqueous solution by peat [J]. Water Air and Soil Pollution, 2004, 158: 77-97.
[25] FAGHIHIAN H, FARSANI S N. Modification of polyacrylamide– β–zeolite composite by phytic acid for the removal of lead from aqueous solutions [J]. Polish Journal of Chemical Technology, 2013, 15: 1-6.
[26] DONG Li-jing, ZHU Zhi-liang, MA Hong-mei, QIU Yan-ling, ZHAO Jian-fu. Simultaneous adsorption of lead and cadmium on MnO2-loaded resin [J]. Journal of Environmental Sciences, 2010, 22: 225-229.
[27] GHEJU M, BALCU I, MOSOARCH G. Removal of Cr(VI) from aqueous solutions by adsorption on MnO2 [J]. Journal of Hazardous Materials, 2016, 310: 270-277.
[28] WANG Zi-meng, LEE S W, CATALANO J G, LEZAMA- PACHECO J S, BARGAR J R, TEBO B M, GIAMMAR D E. Adsorption of uranium(VI) to manganese oxides: X-ray absorption spectroscopy and surface complexation modeling [J]. Environmental Science & Technology, 2013, 47: 850-858.
[29] FREUNDLICH H M F. Over the adsorption in solution [J]. Journal of Physical Chemistry, 1906, 57: 385-471.
[30] ALLEN S J, BROWN P A. Isotherm analyses for single component and multi-component metal sorption onto lignite [J]. Journal of Chemical Technology and Biotechnology, 1995, 62: 17-24.
[31] DONG Li-jing, ZHU Zhi-liang, MA Hong-mei, QIU Yan-ling, ZHAO Jian-fu. Simultaneous adsorption of lead and cadmium on MnO2-loaded resin [J]. Journal of Environmental Sciences, 2010, 22: 225-229.
[32] OBREGON-VALENCIA D, SUN-KOU M D R. Comparative cadmium adsorption study on activated carbon prepared from aguaje (Mauritia flexuosa) and olive fruit stones (Olea europaea L.) [J]. Journal of Environmental Chemical Engineering, 2014, 2: 2280-2288.
[33] WANG Kang, ZHAO Jian-hai, LI Hai-yan, ZHANG Xin-yu, SHI Huan-huan. Removal of cadmium(II) from aqueous solution by granular activated carbon supported magnesium hydroxide [J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 61: 287-291.
冯宁川1,范 玮1,朱美霖1,郭学益2
1. 宁夏医科大学 基础医学院,银川 750004;
2. 中南大学 冶金与环境学院,长沙 410083
摘 要:以黄芪废渣为原料,用KOH为活化剂制备黄芪废渣活性炭,并用KMnO4改性,改性前后的黄芪废渣活性炭用于对水溶液中Cd2+的吸附。采用扫描电子显微术、比表面积测定、X射线衍射、红外光谱和贝母滴定等方法对改性前后的黄芪废渣活性炭进行表征;通过静态吸附实验考察改性前后黄芪废渣活性炭对水溶液中Cd2+的吸附性能。结果表明,KMnO4改性后活性炭表面含氧官能团增加,MnO2沉积到活性炭表面。改性前后的黄芪废渣活性炭对Cd2+的吸附符合准二级动力学方程,等温吸附更符合Langmuir模型,改性前后的黄芪废渣活性炭对Cd2+的饱和吸附量分别是116.96和217.00 mg/g。KMnO4显著改变了黄芪废渣活性炭的物理化学性质和表面结构,改性后的黄芪废渣活性炭对Cd2+的吸附能力明显提高。
关键词:黄芪废渣;活性炭;改性;吸附;Cd2+
(Edited by Bing YANG)
Foundation item: Project supported by West China Top Discipline Program in Basic Medicine Sciences, Ningxia Medical University, China; Project (21266026) supported by the National Natural Science Foundation of China
Corresponding author: Xue-yi GUO; Tel: +86-731-88877863; E-mail: xyguo@csu.edu.cn
DOI: 10.1016/S1003-6326(18)64712-0

