文章编号:1004-0609(2008)S1-0166-06
201×7树脂对铁氰络合物的吸附动力学
兰新哲,李秀玲,宋永辉,张秋利
(西安建筑科技大学 贵金属工程研究所,西安,710055)
摘 要:对201×7树脂吸附铁氰化合物的过程进行动力学研究。结果表明,201×7树脂对铁氰化合物有很好的吸附效果。在298 K时,201×7树脂对亚铁氰化络合离子[Fe(CN)6]4-和铁氰化络合离子[Fe(CN)6]3-的静态饱和吸附量分别为8.620和12.072 mg/mL。用均相颗粒扩散模型和收缩核模型对吸附参数进行描述,表明201×7树脂对[Fe(CN)6]3-和[Fe(CN)6]4-的吸附过程均属于液膜扩散控制。由树脂对[Fe(CN)6]4-和[Fe(CN)6]3-的等温吸附线得到Freundlich常数n分别为4.786和6.145;吸附过程中分离系数S和选择系数K均大于1,表明201×7树脂对[Fe(CN)6]4-和[Fe(CN)6]3-两种络合离子都是容易吸附的,而且[Fe(CN)6]3-离子比[Fe(CN)6]4-离子更容易被吸附。
关键词:201×7树脂;铁氰化物;吸附;动力学
中图分类号:X 703 文献标识码:A
Adsorption kinetics of 201×7 resin for iron cyanocomplexes
LAN Xin-zhe, LI Xiu-ling, SONG Yong-hui, ZHANG Qiu-li
(Institute of Precious Metal Engineering, Xi’an University of Architecture and Technology,Xi’an 710055, China)
Abstract: Kinetics measurements on the adsorption of iron cyanocomplexes with the 201×7 ion-exchange resin were investigated. The results show that the 201×7 resin has good adsorption ability for iron cyanocomplexes. The static saturated adsorptive capacity at 298 K is 8.620 and 12.072 mg/mL for ferrocyanide [Fe(CN)6]4- and ferricyanide [Fe(CN)6]3-, respectively. Analyses of the respective rate data in accordance with homogeneous particle diffusion model and shell progressive model are used to explain the ions adsorption kinetics, which indicates that the controlling step of adsorbing [Fe(CN)6]3- and [Fe(CN)6]4- is liquid film diffusion. Isotherm adsorption curves deduce that Freundlich constant, n, is 4.786 and 6.145 for [Fe(CN)6]4- and [Fe(CN)6]3-, respectively. In adsorption process, the separation factor S and selectivity coefficient K are both greater than 1. The facts indicate that both [Fe(CN)6]4- and [Fe(CN)6]3- are easily adsorbed on 201×7 resin, furthermore, the adsorption of [Fe(CN)6]3- on 201×7 resin is easier than that of [Fe(CN)6]4-.
Key words: 201×7 resin; iron cyanocomplex; adsorption; kinetics
从提金尾液中回收氰化物具有重要的环保和经济价值,目前用离子交换树脂回收氰化物已经迅速发展起来[1]。加拿大FlinFlon矿最早研究了用酸化法回收尾液中的氰化物,酸化过程将氰化物分解为游离氰化物(HCN)和金属离子,再通过挥发和中和工艺可以有效回收氰化物。
但是当尾液中有亚铁氰化物存在时,树脂上就会出现普鲁士蓝沉淀,一般表达式是Me2Fe(CN)6?H2O (Me=Cu, Ni, Zn)。有研究发现这种沉淀会使树脂中毒,而且非常稳定很难分解[2-3]。铁氰化络合离子[Fe(CN)6]3-和亚铁氰化络合离子[Fe(CN)6]4-稳定常数都很高,lg K分别为43.6和35.4,立体化学结构均为八面体,属于强稳定性氰化物。
本文作者主要对201×7树脂吸附两种铁氰络合物的动力学过程进行研究,对实验过程中得到的动力学数据进行图解和数值分析,从而得到吸附速率控制步骤和离子交换过程的动力学参数。这一研究可为树脂上铁氰络合物的有效解吸,解决树脂中毒问题提供一定的理论依据。
1 实验
1.1 材料与设备
实验所用201×7树脂(南开大学提供)经预处理转型为Cl型[4]。
实验所用单纯的铁氰化物溶液和亚铁氰化物溶液分别由相应的金属氰化物盐K3Fe(CN)6(AR)和 K4Fe(CN)6·3H2O(AR)配制而成。混合溶液含有两种铁氰化合物(铁氰化络合离子[Fe(CN)6]3-和亚铁氰化络合离子[Fe(CN)6]4-),且铁氰化络合离子[Fe(CN)6]3-的浓度稍高于亚铁氰化络合离子[Fe(CN)6]4-的浓度。配制的模拟溶液的质量浓度约80.37 mg/L,与提金尾液的组成基本一致。溶液中添加质量分数为2.0×10-6的氰化钾(AR)及氢氧化钾(AR),以调整pH值保持在10.0~10.5之间,溶液中没有沉淀出现。所有的氰化物溶液在使用前均置于容积为1 L的PVC瓶中,冷藏于4 ℃条件下。
实验所用设备有:HH-4恒温水浴锅,78-1磁力加热搅拌器,TG328A电子天平和6800A原子吸收光谱仪。
1.2 吸附和分析方法
量取一定体积的预处理树脂置于烧杯中,加入铁氰化物溶液或亚铁氰化物溶液进行静态平衡吸附实验。在一定温度下将烧杯至于磁力搅拌器中进行搅拌实验。在达到吸附平衡之前不断量取上层澄清溶液,分析铁的浓度[5-6]。吸附容量Q(mg/mL)和吸附率E(%)可由下面的公式计算得到(公式中符号的意义和单位详见文后附录):


溶液中铁的浓度通过原子吸收光谱仪测量。
2 离子交换过程的动力学模型
铁氰化合物在离子交换树脂上的交换反应,同其它的固液间的多项反应一样,可以由一系列的控制速率的步骤来描述:1) 离子扩散通过颗粒周围的液膜(膜扩散(LFD)):2) 离子扩散通过树脂聚合物基体(颗粒扩散控制(PDC)):3) 基体上附着的功能团的化学反应。这3个连续发生的步骤中,通常有一个对离子交换反应的阻力比其它两个要大,所以可以认为该步骤为交换过程的速率控制步骤[7]。这里选用了两个动力学模型来描述201×7树脂对两种铁氰化络合离子的吸附过程,即均相颗粒扩散模型(HPDM)和收缩核模型(SPM),这两个模型已被广泛用于模拟离子交换过程[8-10]。
2.1 均相颗粒扩散模型(HPDM)
在这个模型中,离子交换机理是液相中的[Fe(CN)6]4-或[Fe(CN)6]3-与树脂相中的Cl-克服一系列阻力进行平衡扩散的过程。[Fe(CN)6]4-或[Fe(CN)6]3- 与Cl-进行交换的过程可以严格的用Nernst Plank方程来描述。
离子自溶液进入球形树脂相的颗粒扩散控制过程可以用下面的方程进行描述:
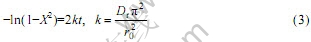
如果离子交换过程是由液膜扩散步骤控制的,可以用下面的方程进行描述:
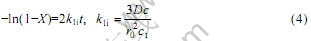
2.2 收缩核模型(SPM)
当聚合物的空隙率比较小、对液体反应物不具有渗透性时,离子交换过过程可以通过收缩核模型进行研究。反应时间和反应程度之间的关系可以由下面的公式进行描述。
树脂对某些离子具有很高的亲和力,吸附过程为不可逆过程,用收缩核模性来描述这一过程是很有效的。因为树脂对[Fe(CN)6]4-和[Fe(CN)6]3-均有很强的亲和力,所以用收缩核模性来描述这一吸附过程的动力学数据。
1) 当吸附过程为液膜扩散控制时,

2) 当吸附过程为颗粒扩散控制时,
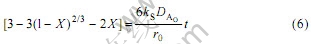
3) 当吸附过程为化学反应控制时,

3 结果与讨论
3.1 静态饱和吸附容量的测定
实验温度为298 K,量取5 mL树脂两份置于不同的烧杯中,分别加入铁氰化溶液和亚铁氰化溶液,取样分析,测得一系列相应时间下树脂的吸附容量,如表1所列。
表1 静态饱和吸附容量的测定
Table 1 Determination of static saturated adsorptive capacity
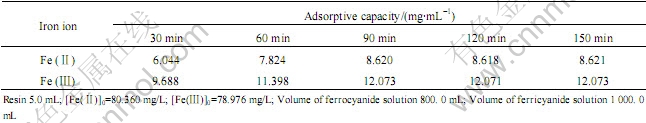
根据表1计算得到201×7树脂对[Fe(CN)6]4-和 [Fe(CN)6]3-离子的静态饱和吸附容量分别为8.620和12.072 mg/mL。
3.2 吸附速率常数以及速率控制步骤的确定
量取5 mL树脂两份置于不同的烧杯中,分别加入铁氰化溶液和亚铁氰化溶液。在吸附过程中,不断量取10 mL上层澄清溶液分析溶液中铁的浓度直至浓度保持不变,进行体积修正得到一系列数据。
根据上述定义的F(X)—t的方程式(3)~(7),将所有实验数据进行图解分析,如图1和图2所示。由图可以看到,由于化学反应和颗粒扩散的实验数据经拟合没有形成一条直线,所以吸附过程不是化学反应和颗粒扩散控制的。而根据式(4)和(5),两种铁氰化离子的数据进行拟合后均为直线,拟合数据如表2和3所列。
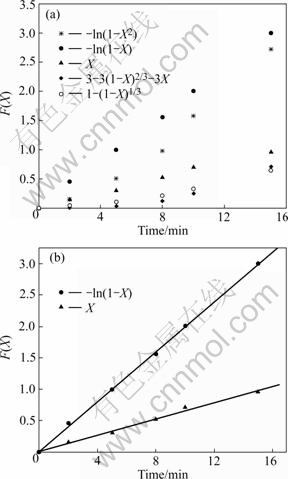
图1 [Fe(CN)6]4-的动力学模型方程F(X)的图解分析
Fig.1 Tests of kinetic model equation F(X) vs time defined by equations for [Fe(CN)6]4- (Resin 5.0 mL; [Fe(Ⅱ)]0=80.037 mg/L; T=298 K; Volume of solution 600.0 mL)
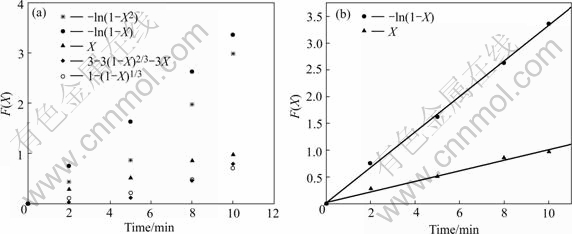
图2 [Fe(CN)6]3-的动力学模型方程F(X)的图解分析
Fig.2 Tests of kinetic model equation F(X) vs time defined by equations for [Fe(CN)6]3- (Resin 5.0 mL; [Fe(Ⅱ)]0=84.690 mg/L; T=298 K; Volume of solution 600.0 mL)
表2 方程-ln(1-X)对时间(t)的线性拟合分析结果
Table 2 Lineal regression analysis of function -ln(1-X) vs time (t)
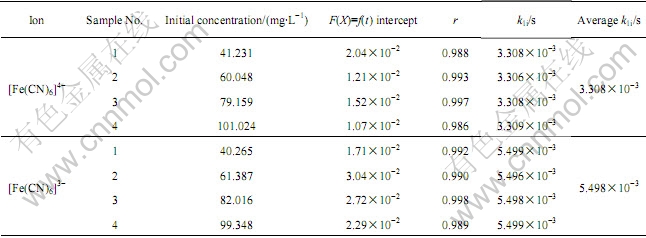
表3 方程X对时间(t)的线性拟合分析结果
Table 3 Lineal regression analysis of function X vs time (t)
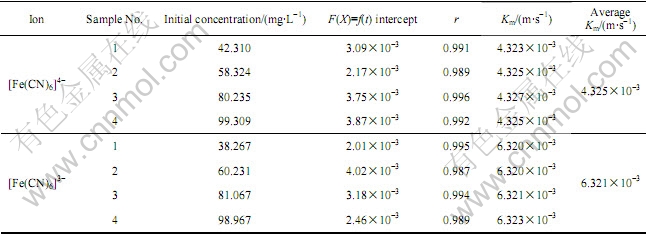
由表2和3中的线性回归结果可以看到,该吸附过程可以用这两个方程进行描述,并通过直线斜率可计算得到物质传递系数Km和吸附速率常数k1i,所以可以确定树脂对[Fe(CN)6]3-和[Fe(CN)6]4-的吸附过程是由液膜扩散为控制步骤的。表2和3中已得到液膜扩散控制的吸附过程中的Km和k1i,对于[Fe(CN)6]4-和[Fe(CN)6]3-离子的吸附速率常数分别为3.308×10-3和5.498×10-3/s,物质传递系数Km分别为4.325×10-3和6.321×10-3 m/s。
3.3 等温吸附曲线
分别量取2、4、6、8和10 mL树脂各两份置于不同的烧杯中,分别加入铁氰化溶液和亚铁氰化溶液,实验条件为:[Fe(Ⅱ)]0=79.267 mg/L,[Fe(Ⅲ)]0=82.015 mg/L,T=298 K,总溶液体积800.0 mL。用著名的Freundlich 等温吸附方程进行线性拟合分析[7]:

当吸附达到平衡时,分别测得剩余氰化物的平衡浓度ρc(mg/L)和相应的吸附容量Q(mg/mL),线性分析如图3所示。
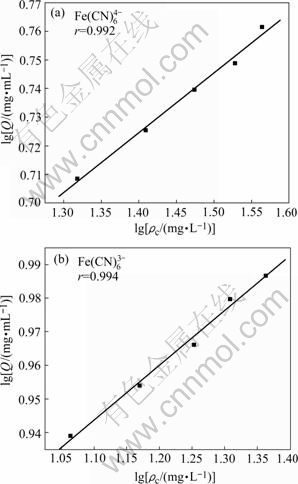
图3 [Fe(CN)6]4-和[Fe(CN)6]3-的Freundlich 等温吸附线
Fig.3 Freundlich isotherm curves of sorption [Fe(CN)6]4- and [Fe(CN)6]3-
Freundlich常数n可表征吸附材料的均一性或吸附反应的强度[11]。当n在2和10之间时,可以认为吸附过程是容易进行的,而n小于0.5时吸附过程是难以进行的。n也可表征优惠吸附,当n大于1时为优惠吸附,等于1时为线性吸附,n小于1时为非优惠吸附。
根据lg Qe—lg ρc线性关系得到树脂对[Fe(CN)6]4-和[Fe(CN)6]3-吸附的 Freundlich常数n值分别为4.768和6.145。当n在2和10之间时,表明201×7树脂对[Fe(CN)6]4-和[Fe(CN)6]3-两种离子的吸附过程均是容易进行的[12]。
3.4 吸附选择性的测定
量取5.0 mL树脂3份置于不同的烧杯中,分别加入含有两种铁氰化离子的模拟溶液。吸附过程中不断量取10 mL上层澄清液,分析测定溶液中铁的浓度直至保持恒定,再进行体积修正得到一系列数据。
根据下面的公式计算得到分离系数SA/B和选择系数KA/B [13],如表4所示。


表4 混合铁氰化溶液中的分离系数S和选择系数K
Table 4 Separation factors, S and selectivity coefficients K derived from mixed iron cyanide solutions
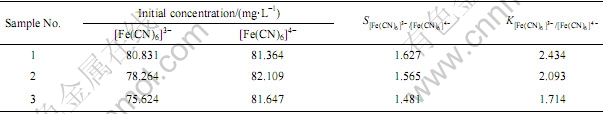
由表4中的数据可以看到分离系数SA/B和选择系数KA/B均大于1,这表明201×7树脂对[Fe(CN)6]3-的吸附较[Fe(CN)6]4-更容易[13]。通过以上实验可以得到树脂对对[Fe(CN)6]3-的吸附速率要比对[Fe(CN)6]4-的吸附快。因为树脂吸附[Fe(CN)6]3-离子需要给出3个功能基进行交换,吸附一个[Fe(CN)6]4-必须同时给出4个功能基,而对于长链的聚乙烯基体来说同时接近并交换4个功能基团是很困难的,所以树脂吸附[Fe(CN)6]3-离子要必吸附[Fe(CN)6]4-要容易[14]。SHARPE[15]提到亚铁氰化离子是不稳定的,容易失去一个价键而氧化成稳定的铁氰化离子。
4 结论
1) 201×7树脂对铁氰络合物有很好的吸附能力。298 K时,[Fe(CN)6]4-和 [Fe(CN)6]3-两种离子的静态饱和吸附容量分别为8.620和12.072 mg/mL。
2) 用颗粒扩散模型和收缩核模型对实验数据进行拟合,结果表明201×7树脂对[Fe(CN)6]4-和 [Fe(CN)6]3-两种离子的吸附过程均是液膜扩散控制。
3) 通过不同浓度的铁氰溶液的吸附实验得到 201×7树脂对[Fe(CN)6]4-和[Fe(CN)6]3-两种离子的吸附速率常数分别为3.308×10-2/s和5.498×10-2/s,物质扩散系数分别为4.325×10-3 m/s和6.321×10-3 m/s。
4) 吸附等温线得到[Fe(CN)6]4-和[Fe(CN)6]3-两种离子的吸附Freundlich常数n分别为4.786和6.145。n的值在2和10之间表明吸附是容易进行的。
5)分离系数S和选择系数K均大于1,表明201×7树脂对[Fe(CN)6]3-的吸附较[Fe(CN)6]4-更容易。
REFERENCES
[1] LEAO V A, CIMINELLI V S T, COSRA R S. Cyanide recycling using strong-base ion exchange resins [J]. Journal of Metals, 1998, 50(10): 71-74.
[2] FERNANDO K, TRAN T, LAING S, KIM K J. The use of ion exchange resins for the treatment of cyanidation tailings (part 1): Process development of selective base metal elution [J]. Minerals Engineering, 2002, 15: 1163-1171.
[3] BACHILLER D, TORRE M, RENDUELES M. Cyanide recovery by ion exchange from gold ore waste effluents containing copper [J]. Minerals Engineering, 2004, 17: 767-774.
[4] 兰新哲, 宋永辉, 廖 赞, 何 敏. 含氰尾液综合回收研究[J]. 稀有金属, 2005, 29(4): 493-497.
LAN Xin-zhe, SONG Yong-hui, LIAO Zan, HE Min. Comprehensive recovery of tail solution containing cyanide [J]. Chinese Journal of Rare Metals, 2005, 29(4): 493-497.
[5] SHU Zeng-Nian, XIONG Chun-hua, WANG Xu. Adsorption behavior and mechanism of amino methylene phosphonic acid resin for Ag(Ⅰ) [J]. Trans Nonferrous Met Soc China, 2006, 16(3): 700-704.
[6] XIONG Chun-hua, SHEN Qiu-xian. Adsorption of Er(Ⅲ) and its mechanism on diglycolamidic acid resin [J]. Journal of Rare Earths, 2002, 20(5): 492-496.
[7] LE?O V A, LUKEY G C. The dependence of sorbed copper and nickel cyanide speciation on ion exchange resin type [J]. Hydrometallurgy, 2001, 61: 105-119.
[8] HELFFERICH F. Ion exchange [M]. New York: McGraw-Hill, 1962.
[9] PETRUZZELLI D, LIBERTI L, PASSINO R, HELFFERICH G, HWANG Y L. Chloride/sulfate exchange kinetics: solution for combined film and particle diffusion control [J]. React Polym, 1987, 5: 219-227.
[10] CORTINA J L, WARSHAWSKY A, KAHANA N, KAMPEL V, SAMPAIO C H, KAUTZMANN R M. Kinetics of goldcyanide extraction using ion-exchange resins containing piperazine functionality [J]. Reactive & Functional Polymers, 2003, 54: 25-35.
[11] GONZALEZ-LUQUE S, STREAT M. Uranium sorption from phosphoric acid solutions using selective ion exchange resins (part II): Kinetic studies [J]. Hydrometallurgy, 1983, 11: 227-245.
[12] MILTZAREK G L, SAMPAIO C H, CORTINA J L. Cyanide recovery in hydrometallurgical plants: Use of synthetic solutions constituted by metallic cyanide complexes [J]. Minerals Engineering, 2002, 15: 75-82.
[13] HAINEY1 P, SHERRINGTON D C. Oligoamine-functionalised poly(glycidyl methacrylate-ethyleneglycol dimethacrylate) resins as moderate base extractants for gold from cyanide solutions [J]. Reactive & Functional Polymers, 2000, 43: 195-210.
[14] LE?O V A, LUCKY G C. The effect of resin structure on the loading of copper and iron cyanocomplexes [J]. Solvent Extraction and Ion Exchange, 2001, 19(3): 507-530.
[15] SHARPE A G. The chemistry of cyano complexes of the transition metals [M]. London: Academic Press Inc, 1976.
附录
a0
化学计量系数;
c
两种交换物质的总浓度,mol/L;
cr
离子交换介质中两种交换物质的总浓度,mol/L;
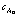
溶液中物质A的浓度,mol/L;
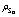
树脂中未反应物质的浓度,mg/L;
D
液相中的扩散系数,m2/s;
Dr
固相中的扩散系数,m2/s;
k1i
液膜扩散速率常数(大量溶液);
X
交换度;
De,r
固相中的扩散系数,m2/s;
Km
物质通过液膜的传递系数,m/s;
kS
表面反应常数,m/s;
ρ0
溶液中铁的初始浓度,mg/L;
ρe
溶液中铁的平衡浓度,mg/L;
V
溶液的总体积,mL;
Vr
树脂的体积,mL;
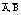
树脂相中的A、B的铁的浓度;
a, b
络合离子A 和 B 的电荷数(价态);
Q
吸附容量,mg/mL;
ρc
剩余氰化物的平衡浓度,mg/L;
K,n
Freundlich 常数。
通讯作者:宋永辉;副教授;电话:029-82201248;E-mail: syh1231@126.com
(编辑 何学锋)