文章编号:1004-0609(2010)08-1636-09
硫化锌氧压浸出过程的φ—pH图
牟望重1, 2,张廷安1, 2,吕国志1, 2,古 岩1, 2,豆志河1, 2
(1. 东北大学 材料与冶金学院,沈阳 110819;
2. 东北大学 多金属共生矿生态化利用教育部重点实验室,沈阳 110819)
摘 要:通过热力学计算,得到ZnS-H2O系各物质高温条件下的GΘ值、φΘ值或pHΘ值以及各反应对应的电位表达式,分别绘制出不同离子活度、不同氧分压条件下的φ—pH图。由φ—pH图可以看出,随着离子活度的增大,S与Zn2+稳定区逐渐增大,但构成稳定区各反应的pH值上下限范围逐渐减小;随着氧分压的增大,水的稳定区逐渐增大。对ZnS进行了氧压浸出实验研究,采用全谱直读等离子光谱仪(ICP)、XRD和XRF等方法对浸出液和浸出渣进行分析、表征。结果表明:当初始酸浓度为15%、氧分压为1.1 MPa、浸出温度为160 ℃、液固比为8:1、浸出时间为90 min、搅拌速度480 r/min时,锌的浸出率为98.86%,硫的转化率为81.33%。实际结果与根据φ—pH图理论计算结果吻合。
关键词:氧压浸出;ZnS-H2O系;φ—pH图;氧分压;离子活度
中图分类号:TF803.21 文献标志码:A
φ—pH figure during oxidative pressure leaching of zinc sulfide
MU Wang-zhong1, 2, ZHANG Ting-an1, 2, L? Guo-zhi1, 2, GU Yan1, 2, DOU Zhi-he1, 2
(1. School of Materials and Metallurgy, Northeastern University, Shenyang 110819, China;
2. Key Laboratory of Ecological Utilization of Multi-metal Intergrown Ores, Ministry of Education,
Northeastern University, Shenyang 110819, China)
Abstract: The values of GΘ, φΘ or pHΘ and relevant potential expressions were obtained through thermodynamical calculation, and φ—pH figures of ZnS-H2O system at different ionic activities and different oxygen partial pressures were obtained. With increasing the ionic activity, the stable regions of S and Zn2+ in the φ—pH figures become gradually large, but the limit of pH in the stable region becomes gradually small. With increasing the oxygen partial pressure, the stable region of H2O become large. The oxidative pressure leaching experiments of ZnS were done in autoclave, the leaching solution and leaching slag were characterized by inductively coupled plasma-atomic emission spectrometry (ICP), XRD and XRF. The results indicate that when the sulfuric acid concentration is 15%, oxygen partial pressure is 1.1 MPa, leaching temperature is 160 ℃, liquid-solid ratio is 8?1, leaching time is 90 min and stirring rate is 480 r/min, the leaching rate of zinc is 98.86% and the conversion rate of sulfur is 81.33%. The experiment results well accord to the calculation results of φ—pH figures.
Key words: oxidative pressure leaching; ZnS-H2O system; φ—pH figure; oxygen partial pressure; ionic activity
锌冶金分为火法和湿法两种工艺,传统的湿法炼锌工艺是火法-湿法的联合过程。在传统的湿法工艺中,硫化锌精矿要先行焙烧脱硫,会产生大量SO2气体,造成对气环境的污染[1-3]。硫化锌氧压浸出技术起源于加拿大,Sherrit Gordon公司与Cominco公司于1981年在Trail建立了世界上第一个锌精矿氧压浸出厂;1983年,第二个锌精矿氧压浸出厂——Timmins厂建成投产;1991年,德国Ruhr锌厂建成;1993年,加拿大Hudson Bay Mining and Smelting公司建成了世界上第一个两段氧压浸出锌冶炼厂;2002年,哈萨克斯坦引进加拿大Cominco公司的氧压浸出技术并已顺利投产;2004年,我国云南冶金集团建成了我国第一个锌精矿氧压浸出厂,年产电锌10 000 t[4-5]。相比于传统的湿法炼锌工艺,该工艺具有浸出条件简单、工艺流程短、产生“三废”少、生产成本低、锌浸出率高等一系列优点,该技术最主要的优势在于将硫化锌中的硫元素转化为单质硫,易于回收、储存和运输[6-7],硫化锌氧压浸出的基本反应式如式(1)所示。在氧压浸出过程中,硫化锌中的S元素在不同的酸浓度以及氧分压等条件下分别会形成SO42-、S单质以及H2S等产物,因此,控制酸浓度及氧分压等条件对保证单质硫的转化以及锌的浸出是十分必要的[8-9]。
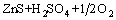 =
=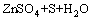 (1)
(1)
φ—pH图是研究硫化锌氧压浸出过程重要的热力学依据,但目前可供参考的硫化物-水系的φ—pH图多是在常温常压下(25 ℃、0.1 MPa)绘制的,对于高温高压体系下硫化物-水系φ—pH图的相关研究较少,王吉昆等[10]只在150 ℃时对高铁闪锌矿的φ—pH图进行研究。本文作者在高温及氧压条件下,通过热力学计算绘制出ZnS-H2O系的φ—pH图,分析离子活度和氧分压等因素变化对硫化锌氧压浸出过程的影响,同时以分析纯ZnS为原料进行氧压浸出单因素实验,考察不同酸浓度及氧分压条件下锌浸出率及硫转化率的变化情况,并验证理论计算的结果。
1 热力学计算方法
计算不同温度下各物质的GTΘ值可以采用平均热熔法、离子近似线性热熔法等[11],但计算过程十分复杂。易宪武[12]在研究高温As-H2O体系时,根据任意温度下的GTΘ表达式,结合CRISS和COBBLE[13]提出的“熵的对应原理”与ХОДАКОВСКИЙ[14]提出的计算绝对熵的经验公式,通过近似计算,得出GTΘ的计算公式如式(2)所示。


 (2)
(2)
其中,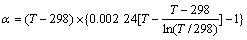

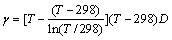
式中各温度下A、D值及α、β、γ值见文献[11];Z代表离子反应的电荷数。
在湿法冶金过程中,可用式(3)表示反应过程:
aA+mH++Ze=bB+cH2O (3)
根据Nernst等温方程式,其平衡电势表达式如式(4)或式(5)所示。
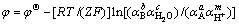 (4)
(4)
 (5)
(5)
根据 ,只要知道反应中的
,只要知道反应中的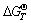 、K和φΘ中的任何一个值,就可由式(5)求出平衡电位,绘制出该温度下的φ—pH图。
、K和φΘ中的任何一个值,就可由式(5)求出平衡电位,绘制出该温度下的φ—pH图。
2 高温ZnS-H2O系φ—pH图的绘制
各物质在常温常压下的热力学数据来自文献[15-16],根据式(2)的方法计算出高温条件下ZnS-H2O系内各物质的GTΘ值,通过 来计算各反应高温条件下的ΔGTΘ值。将ΔGTΘ代入公式φΘ=-ΔGΘ/RT,即可求出不同温度下各反应平衡电位的表达式,进而绘制出ZnS-H2O系在不同活度和不同氧分压条件下的φ—pH图。在确定的温度条件下,通过比较φ—pH图中各离子稳定区的变化,分析氧分压与离子活度对浸出过程的影响规律。
来计算各反应高温条件下的ΔGTΘ值。将ΔGTΘ代入公式φΘ=-ΔGΘ/RT,即可求出不同温度下各反应平衡电位的表达式,进而绘制出ZnS-H2O系在不同活度和不同氧分压条件下的φ—pH图。在确定的温度条件下,通过比较φ—pH图中各离子稳定区的变化,分析氧分压与离子活度对浸出过程的影响规律。
2.1 不同离子活度条件下ZnS-H2O系的φ—pH图
在水溶液电化学中,活度是一个很重要的概念,离子活度与浓度的关系可表示为 。根据德拜-休克尔极限公式以及Guggenheim提出的改善方法,黄子卿[17]在大量实验数据基础上总结出了计算电解质活度系数的完全经验公式,如式(6)所示。分别取ZnS-H2O体系中各离子的浓度为2.0、1.0、0.1、0.01 mol/L,乘以活度系数即可得到该体系中各离子的离子活度。表1所列为ZnS-H2O体系中各离子的离子强度I以及离子活度γ的计算值。
。根据德拜-休克尔极限公式以及Guggenheim提出的改善方法,黄子卿[17]在大量实验数据基础上总结出了计算电解质活度系数的完全经验公式,如式(6)所示。分别取ZnS-H2O体系中各离子的浓度为2.0、1.0、0.1、0.01 mol/L,乘以活度系数即可得到该体系中各离子的离子活度。表1所列为ZnS-H2O体系中各离子的离子强度I以及离子活度γ的计算值。
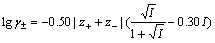 (6)
(6)
在浸出过程中,考察离子活度的影响可以表现在考察溶液的酸浓度上。在确定温度为120 ℃,氧分压为0.8 MPa的条件下,改变离子浓度分别为2.0、1.0、0.1、0.01 mol/L,根据Nernst等温方程式,即可求出120 ℃、0.8 MPa条件下各反应的φΘ值或pH值,如表2所列。将表1中的γ值和表2中的φΘ值或pH值代入式(5),即可得到在温度为120 ℃、氧分压为0.8 MPa、对应离子浓度分别为2.0、1.0、0.1、0.01 mol/L时不同离子活度的ZnS-H2O系的φ—pH图,如图1所示。将相同温度、相同氧分压、不同活度条件下的φ—pH图进行叠加,得到Zn2+与S稳定区的变化趋势如图2所示。
由图1与2可知,Zn2+与S的稳定区边界对应着生成单质硫与浸出锌反应pH值上限与下限,因此只要控制浸出过程的酸浓度保持在pH的上下限范围之内,就可以保证生成S与Zn2+的反应顺利进行。以120 ℃为例,按照式(7)~(9)计算出pH值的上下限,结果列于表3中。
通过比较可以发现,随着离子浓度由0.01 mol/L增大到2.0 mol/L,φ—pH图中对应的Zn2+与S稳定区的面积逐渐增大,但对应的pH值的上下限范围越来越小。这说明随着离子活度的增大,反应生成Zn2+与S的趋势越来越稳定,但对酸浓度条件要求越来越高。
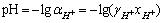 (7)
(7)
 (8)
(8)
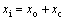 (9)
(9)
式中:xi、xo、xc为酸的初始的浓度、结束时的浓度及反应中消耗的浓度。
2.2 不同氧分压条件下ZnS-H2O系的φ—pH图
在考察了不同活度下ZnS-H2O系φ—pH图的基础上,考察不同氧分压条件下φ—pH图的变化趋势。经过热力学计算,将160 ℃、对应各离子浓度为1.0 mol/L时的离子活度条件下,将ZnS-H2O系中所有反应的平衡电位表达式列于表4中。氧分压的变化,主要改变φ—pH图中氧标线的位置,即改变φ—pH图中水稳定区的范围。将不同氧分压下氧标线对应反应的平衡电位变化值列于表5中,根据表4与5的数据,绘制出温度为160 ℃、对应各离子浓度为1.0 mol/L的离子活度、氧分压分别为0.2、0.5、0.8、1.1 MPa的 φ—pH图,如图3所示。
比较相同温度、相同活度、不同氧分压条件下ZnS-H2O系的φ—pH图可以发现,增大氧分压会使氧标线上移,增大了水的稳定区,在同一电位下增大氧分压降低了浸出过程析出O2的可能性,保证了水溶液中的各反应能够在更高的平衡电位下顺利进行。同时从反应动力学的角度来说,水溶液中溶解氧的存在可以加速固体硫化锌在浸出液中的溶解,从而促进反应的进行,在单位时间内提高锌的浸出率与硫的转 化率。
3 实验
3.1 原料
实验所使用的原料为分析纯ZnS,浸出液由98%的浓H2SO4按实验参数稀释至所需浓度。
表1 ZnS-H2O体系各物质的离子强度与活度系数计算值
Table 1 Calculated values of ionic strength and ionic activity coefficient of ZnS-H2O system

表2 在120 ℃、0.8 MPa及不同离子活度条件下ZnS-H2O系中各反应的标准电位计算值
Table 2 Calculated values of φΘ of ZnS-H2O system at 120 ℃, 0.8 MPa and different ionic concentrations

表3 S与Zn2+稳定区的初始酸浓度范围
Table 3 Initial acid concentration range in S and Zn2+ stable regions (120 ℃)

3.2 浸出实验
使用KCFD2-10型高压反应釜对硫化锌的氧压浸出过程进行单因素实验研究,分别考察在浸出温度120 ℃、氧分压0.8 MPa、液体体积(mL)与固体质量(g)比8?1、反应时间90 min、搅拌速度360 r/min、初始浓酸浓度10%~20%,以及浸出温度160 ℃、初始酸浓度15%、液体体积(mL)与固体质量(g)比8?1、反应时间90 min、搅拌速度480 r/min、氧分压0.2~1.1 MPa条件下各因素对氧压浸出效果的影响,锌浸出率及硫转化率的计算公式如式(10)和(11)所示。
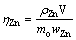 (10)
(10)
式中:ηZn为锌浸出率;mo为加入样品的质量;wZn为原矿中Zn的含量;ρZn为浸出液中Zn2+的质量浓度;V为浸出液体积。
 (11)
(11)
式中:ηS为硫转化率;mo为加入样品的质量; 为原矿中S的含量;
为原矿中S的含量;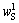 为浸出渣中S的含量;
为浸出渣中S的含量;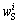 为浸出渣中与各元素结合的非单质S含量;mS为浸出渣质量。
为浸出渣中与各元素结合的非单质S含量;mS为浸出渣质量。
3.3 检测分析
使用美国Leeman公司的Prodigy XP型全谱直读等离子光谱仪(ICP)对浸出液中的Zn2+含量进行检测;使用荷兰帕纳克公司PW3040/60型X射线衍射仪(铜靶,衍射角范围5?<2θ<90?,扫描速率为7 (?)/s)以及日本理学3070e型X荧光光谱仪对浸出渣物相及含量进行分析。
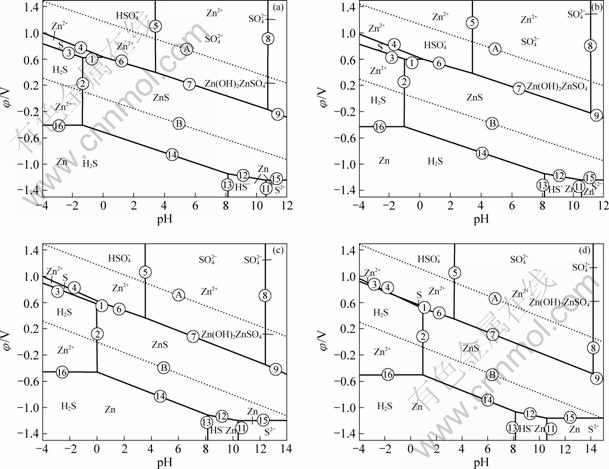
图1 在120 ℃、0.8 MPa及不同活度下ZnS-H2O系的φ—pH图
Fig.1 φ—pH figures of ZnS-H2O system at 120 ℃, 0.8 MPa and different activities: (a) 2.0; (b) 1.0; (c) 0.1; (d) 0.01
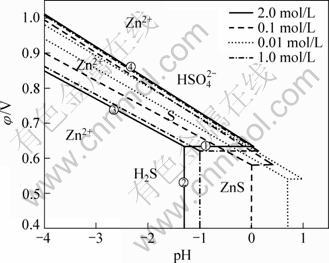
图2 在120 ℃、0.8 MPa及不同离子浓度条件下S与Zn2+稳定区的比较
Fig.2 Comparison of S and Zn2+ stable regions at 120 ℃, 0.8 MPa and different ionic concentrations
4 结果与讨论
4.1 酸浓度对浸出效果的影响
通过比较不同离子活度的φ—pH图,可以确定S与Zn2+稳定区以及pH上下限变化范围。在浸出温度120 ℃、氧分压0.8 MPa、液体体积(mL)与固体质量(g)比8?1、浸出时间90 min、搅拌速度360 r/min的条件下,不同酸浓度条件下ZnS氧压浸出实验结果图4所示。由图4可知,随着酸浓度的增加,锌浸出率及硫转化率呈增大趋势,在酸浓度达到15%左右达到最大值,而后呈下降趋势。此时,锌浸出率为90.86%,硫转化率为70.58%。
表4 160 ℃时ZnS-H2O系中的反应式及φ—pH表达式
Table 4 Reaction and expression of φ—pH of ZnS-H2O system at 160 ℃

表5 160 ℃时ZnS-H2O系不同氧分压下氧标线反应的电位表达式
Table 5 φ expressions of oxygen graticule of ZnS-H2O system at different oxygen partial pressures and 160 ℃

在酸浓度高于15%的条件下,锌的浸出率及硫的转化率下降的主要原因是随着H2SO4浓度的增大,氧在水溶液中的溶解度降低[18],而溶解氧作为硫化锌氧压浸出的反应物,其浓度下降,导致锌的浸出与硫的氧化不能充分进行。同时,硫酸浓度过大,造成水溶液中各离子的活性降低,反应速率减慢,在相同的时间内,氧压浸出反应进行得不完全,故锌浸出率和锌转化率有降低趋势。
4.2 氧分压对浸出效果的影响
在浸出过程中,氧是作为一种重要的反应物而被引入浸出系统的。由于S/S2-的标准还原电位比Zn2+/Zn、Fe3+/Fe2+等的低得多,所以从热力学观点来看,氧对S2-的氧化使得溶液中的S2-浓度被控制在一个很低的范围,从而保证硫元素以单质形态析出的比例较高。同时液固体系中气相氧的存在可以加速固体硫化锌在浸出液中的溶解,促进多相反应的动力学行为。

图3 在160 ℃、离子浓度1.0 mol/L时不同氧分压条件下ZnS-H2O系的φ—pH图
Fig.3 φ—pH figures of ZnS-H2O system with ionic concentration of 1.0 mol/L at 160 ℃ under different oxygen partial pressures: (a) 0.2 MPa; (b) 0.5 MPa; (c) 0.8 MPa; (d) 1.1 MPa
在浸出温度160 ℃、浸出时间90 min、液体体积(mL)与固体质量(g)比8?1、初始浓酸浓度15%、搅拌速度480 r/min时,不同氧分压条件下ZnS氧压浸出结果如图5所示。由图5可以发现,随着氧分压的增大,Zn浸出率增加趋势较小;S转化率提高明显,在氧分压增加到0.8 MPa后,结果趋于稳定。此时锌浸出率为98.86%,硫转化率为81.33%。
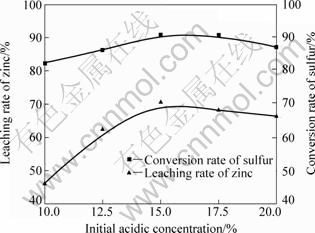
图4 初始酸浓度对ZnS浸出率和转化率的影响
Fig.4 Effects of initial acid concentration on leaching rate and conversion rate
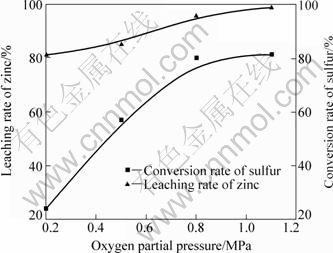
图5 氧分压对ZnS对浸出率和转化率的影响
Fig.5 Effects of oxygen partial pressure on leaching rate and conversion rate
通过比较不同氧分压条件下的φ—pH图,在 160 ℃、氧分压从0.2 MPa增加到1.1 MPa时,水的稳定区逐渐增大,保证溶液中各反应能够在更高的平衡电位下顺利进行而不析出O2。同时根据Henry定律Cb=pKb,溶液中的溶解氧浓度与气相中的氧分压保持一定的平衡关系,即气相中的氧分压越大,液相中溶解氧浓度就越大。因此,增大氧分压能够加速固体硫化锌在浸出液中的溶解,提高氧化速度,增大反应的氧化电位。从硫化锌氧压浸出实验结果可以看出,增大氧分压对反应的热力学变化趋势影响较小,但增大了反应速率,促进了反应的动力学过程。
5 结论
1) 绘制出温度120 ℃,氧分压0.8 MPa,对应离子浓度分别为2.0、1.0、0.1、0.01 mol/L的不同离子活度条件下以及温度160℃,对应离子浓度1.0 mol/L的离子活度,氧分压分别为0.2、0.5、0.8、1.1 MPa条件下的ZnS-H2O系φ—pH图。
2) 比较不同离子活度以及不同氧分压条件下φ—pH图中S与Zn2+稳定区的变化趋势,可以发现随着离子活度的增大,S与Zn2+稳定区逐渐增大,但是构成稳定区各反应的pH值上下限范围逐渐减小;随着氧分压的增大,水的稳定区逐渐增大。
3) 从硫化锌氧压浸出实验结果可以看出,在120℃,当初始酸浓度由10%提高到20%时,Zn浸出率与S转化率先增大后减小,当酸浓度为15%时取得最大值,此时Zn浸出率为90.86%,S转化率为70.58%;在160 ℃,当氧分压由0.2 MPa增大到1.1 MPa时,Zn浸出率增加到98.86%,S转化率增加到81.33%。实验结果与φ—pH图的变化趋势吻合。
REFERENCES
[1] 徐志峰, 邱定蕃, 王海北. 煤在铁闪锌矿氧压酸浸中的应用[J]. 中国有色金属学报, 2008, 18(5): 939-945.
XU Zhi-feng, QIU Ding-fan, WANG Hai-bei. Application of coal in oxidative pressure leaching of marmatite concentrates[J]. The Chinese Journal of Nonferrous Metals, 2008, 18(5): 939-945.
[2] ?OPUR M, ?ZMETIN C, ?ZMETIN E, KOCAKERIM M M. Optimization study of the leaching of roasted zinc sulphide concentrate with sulfate acid solutions[J]. Chemical Engineering and Processing, 2004, 43: 1007-1014.
[3] SAHU S K, SAHU K K, PANDEY B D. Leaching of zinc sulfide concentrate from the ganesh-himal deposit of nepal[J]. Metallurgical and Materials Transactions B, 2006(8): 541-549.
[4] 高良宾, 赫冀成, 徐红江. 硫化锌精矿高温高压浸出技术[J]. 有色矿冶, 2007(4): 33-36.
GAO Liang-bin, HE Ji-cheng, XU Hong-jiang. The technology of zinc sulphide concentrate in high temperature and high pressure leaching[J]. Nonferrous Mining and Metallurgy, 2007(4): 33-36.
[5] 王吉昆, 周廷熙. 硫化锌精矿加压酸浸技术及产业化[M]. 北京: 冶金工业出版社, 2008: 16-17.
WANG Ji-kun, ZHOU Ting-xi. Technology and industrialization of zinc concentrates oxygen pressure leaching [M]. Beijing: Metallurgical Industry Press, 2008: 16-17.
[6] 刘俊峰, 易平贵, 黄可龙. 常压酸浸闪锌矿的条件对锌浸出率的影响[J]. 中国有色金属学报, 2000, 10(5): 728-731.
LIU Jun-feng, YI Ping-gui, HUANG Ke-long. Effects of conditions of soaking blende with sulfuric acid on rate of leaching zinc in oxygen at normal pressure[J]. The Chinese Journal of Nonferrous Metals, 2000, 10(5): 728-731.
[7] HAAKANA T, LAHTINEN M, TAKALA H, RUONALA M, TURUMEN I. Development and modelling of a novel reactor for direct leaching of zinc sulphide concentrates[J]. Chemical Engineering Science, 2007, 62: 5648-5654.
[8] SOUZA A D, PINA P S, LEAO V A, SILVA C A, SIQUERIRA P F. The leaching kinetics of a zinc sulphide concentrate in acid ferric sulphate[J]. Hydrometallurgy, 2007, 89: 72-81.
[9] XIE Ke-qiang, YANG Xian-wan, WANG Ji-kun, YAN Jiang-feng, SHEN Qing-feng. Kinetic study on pressure leaching of high iron sphalerite concentrate[J]. Transaction of Nonferrous Metals Society of China, 2007, 17: 187-194.
[10] 王吉坤, 李存兄, 李 勇, 张红耀, 黄 卉, 阎江峰, 刘 露, 魏 昶. 高铁闪锌矿高压酸浸过程中ZnS-FeS-H2O系的电 位—pH图[J]. 有色金属: 冶炼部分, 2006(2): 2-5.
WANG Ji-kun, LI Cun-xiong, LI Yong, ZHANG Hong-yao, HUANG Hui, YAN Jiang-feng, LIU Lu, WEI Chang. The ε—pH figure of ZnS-FeS-H2O system during acid leaching under pressure of high iron sphalerite[J]. Nonferrous Metals: Extractive Metallurgy, 2006(2): 2-5.
[11] 金哲男, 蒋开喜, 魏绪钧, 王海北. 高温As-S-H2O系电位—pH图[J]. 矿冶, 1999, 8(4): 45-50.
JIN Zhe-nan, JIANG Kai-xi, WEI Xu-jun, WANG Hai-bei. Potential—pH diagrams of As-S-H2O system at high temperature[J]. Mining and Metals, 1999, 8(4): 45-50.
[12] 易宪武. 某些络阳离子标准熵的计算和高温As-H2O体系电位—pH图[J]. 昆明工学院学报, 1982(3): 58-73.
YI Xian-wu. An empirical estimation of standard entropy for some complex cations and the E-pH diagrams of As-H2O system at elevated temperature[J]. Journal of Kunming University of Science and Technology, 1982(3): 58-73.
[13] CRISS C M, COBBLE J W. The thermodynamic properties of high temperature aqueous solutions. Ⅳ. Entropies of the ions up to 200 ℃ and the correspondence principle[J]. Journal of the American Chemical Society 1964, 86(24): 5385-5390.
[14] ХОДАКОВСКИЙ И Л. Migration form of tungsten in hydrothermal[J]. Journal of Chemistry, 1968(12): 1486-1503.
[15] 杨显万. 高温水溶液热力学数据计算手册[M]. 北京: 冶金工业出版社, 1983: 523-674.
YANG Xian-wan. Handbook of thermodynamic data in squeous solutions at high temperature[M]. Beijing: Metallurgical Industry Press, 1983: 523-674.
[16] 傅崇说. 冶金溶液热力学原理与计算[M]. 北京: 冶金工业出版社, 1989: 154-202.
FU Cong-yue. Thermodynamic principle and calculation in metallurgical solutions[M]. Beijing: Metallurgical Industry Press, 1989: 154?202.
[17]黄子卿. 电解质溶液理论导论[M]. 北京: 科学出版社, 1983: 83?87.
HUANG Zi-qing. Introduction of electrolyte solutions theory[M]. Beijing: Science Press, 1983: 83?87.
[18]马荣骏. 湿法冶金原理[M]. 北京: 冶金工业出版社, 2007: 340?341.
MA Rong-jun. Principle on hydrometallurgy[M]. Beijing: Metallurgical Industry Press, 2007: 340?341.
(编辑 李艳红)
基金项目:国家重点基础研究发展计划资助项目(2007CB613504);辽宁省优秀青年科技人才基金资助项目(2005221012);教育部高校博士点专项基金资助项目(20050145029)
收稿日期:2009-10-09;修订日期:2010-04-09
通信作者:张廷安,教授,博士;电话:024-83687732;E-mail:mwz_01@163.com;zta2000@163.net