硅酸镍在氨-硫酸铵-水溶液中的溶解机理
刘智勇,李启厚,刘志宏,杨天足,姜波,李玉虎
(中南大学 冶金与环境学院,湖南 长沙,410083)
摘要:对不同结构的NiSiO3在氨性体系的溶解平衡进行研究,从晶体结构方面考察氧化镍矿在氨性体系中的溶解机理,进一步查明氧化镍中矿物结构对镍浸出行为的影响。研究结果表明:随着温度的升高,NiSiO3的结晶度逐渐增加、晶粒尺寸逐渐增大、微观应变逐渐减小、晶体结构更完整、稳定性更高。在总氨浓度3.5 mol/L,n(NH3)/n(NH4+)为2:3,温度为25 ℃条件下,结晶度为0%和100%的NiSiO3在NH3-(NH4)2SO4-H2O体系中溶解平衡时,溶液中的Ni2+质量浓度分别为7.000 g/L和0.029 g/L,结晶完整的NiSiO3很难溶解。
关键词:硅酸镍;结晶度;晶粒尺寸;微观应变;溶解度
中图分类号:TF803.21;TF813 文献标志码:A 文章编号:1672-7207(2013)07-2669-06
Dissolution behavior of nickel silicate in ammonia-ammonium sulfate aqueous solution
LIU Zhiyong, LI Qihou, LIU Zhihong, YANG Tianzu, JIANG Bo, LI Yuhu
(College of Metallurgical and Environment, Central South University, Changsha 410083, China)
Abstract: The dissolution equilibrium of NiSiO3 with various structures in ammonia system was studied. The dissolution mechanism of nickel oxide ore in ammonia system was examined in terms of crystal structure. The influence of mineral structure of nickel oxide on the leaching of nickel was researched. The results show that with the increase of the temperature, the crystallinity and the grain size of NiSiO3 increase gradually. While the strain decreases, the crystal structure will be more completed, that is, the crystal has higher stability. When the total ammonia concentration is 3.5 mol/L, n(NH3)/n(NH4+) is 2:3, temperature is 25 ℃, the mass concentrations of NiSiO3 with crystallinity of 0% and 100% are 7.000 g/L and 0.029 g/L, respectively. So NiSO3 with ideal structure is difficult to dissolve.
Key words: nickel silicate; crystallinity; crystallite size; strain; solubility
我国镍资源总量828万t,其中,目前技术难以利用的镍资源量638万t,为高碱性脉石型低品位硫化镍矿和氧化镍矿(红土矿),同时伴生铜金属量500万t左右。这类矿石的突出特点是碱性脉石含量高和有价金属赋存状态复杂[1-2]。我国红土氧化镍矿品位低,成分复杂,镁、硅含量(质量分数)高达30%以上,矿物中各种金属离子相互镶嵌,采用火法处理镍回收率低、生产成本高。直接湿法提取时,过程相当复杂和缓慢,铁镁大量溶出造成溶出成本高、有价金属分离过程镍损失大。红土镍矿还原焙烧过程由于铁尖晶石与橄榄石相互镶嵌与互溶,镍与矿物结合紧密,镍分离异常困难[3-5]。红土镍矿硫酸加压浸出、常压浸出或堆浸能显著提高镍、钴的浸出率,并依据浸出液中镍分离富集的工艺组合、萃取体系、溶剂萃取介质的不同,相应出现了多种工艺。例如,Murrin法用H2S沉淀镍、钴;Bulong法将浸出液直接采用Cyanex272萃取分离Co/Ni;Goro法在较低的pH(pH=1~2)下萃取Co,Ni,Zn;Cawse法采用氢氧化物沉淀-氨浸-萃取回收Ni,Co;采用硫酸溶液堆浸-硫化钠沉淀技术处理云南元江的红土镍矿。这些技术用于处理低品位氧化镍矿已实现工业应用,但存在投资大、处理量大、消耗高、资源利用率低、废弃物多及环境负担严重等问题[1, 6-10]。离析法实际是选矿中一种对矿石预处理的方法,适宜处理一些低品位、难选氧化矿或硅酸盐矿石,在低品位铜矿中已经得到应用,但是在处理低品位氧化镍矿还处于研究阶段,尚未成熟[11-16]。氨浸法具有原料适应性广、净化负担轻、工艺流程短等优点,目前正被广大科研工作者所重视,已有一些用氨浸法处理低品位氧化镍矿的报道[17-18]。在此,本文作者对不同结构的NiSiO3在氨性体系的溶解平衡进行研究,从晶体结构方面考察氧化镍矿在氨性体系中的溶解机理,进一步查明氧化镍中矿物结构对镍浸出行为的影响。
1 实验
1.1 实验原料
采用化学沉淀法制备NiSiO3。向1 mol/L NiSO4溶液中以10 mL/min的速度加入1 mol/L Na2SiO3溶液,Na2SiO3为理论量的1.1倍,反应温度为90 ℃,反应时间2 h,生成的沉淀用去离子水洗涤8次,酒精洗涤2次,在300 ℃下干燥24 h,得到NiSiO3。
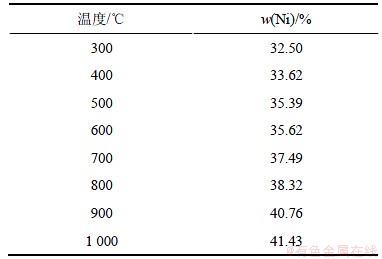
合成的NiSiO3热重-差热分析结果如图1所示。DSC结果表明,在753.6 ℃和939.2 ℃有2个放热峰。热重曲线上,在600 ℃前都有缓慢质量损失,质量损失率达19.1%,但是差热曲线上并没有明显的放热、吸热峰,这可能是由于合成时样品晶体结构内吸附了大量的自由水。在753.6 ℃和939.2 ℃产生的放热峰可能是由非晶态向晶态转变而引起的,这一现象与某些硅铝酸盐的高温放热行为有些类似,如蒙脱石、褐绿脱石在950 ℃附近因非晶态结晶成石英、堇青石、顽辉石而出现放热峰,又如钠沸石、钙沸石、菱沸石等沸石类矿物在1 000 ℃左右因非晶态物质结晶成钙长石而形成放热峰。不同温度下获得的样品的Ni含量(质量分数)如表1所示。从表1可知:300 ℃下获得样品Ni含量为32.50%远低于NiSiO3中Ni的理论含量(43.57%)。这是由于含结晶水或水解生产少量硅酸所致,随着温度的升高,镍含量逐渐增加,接近理论值。
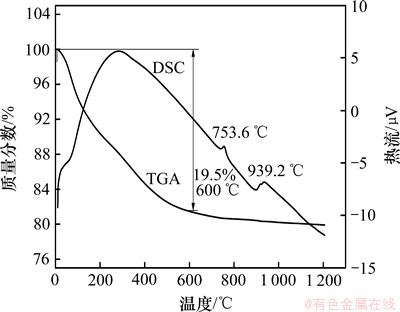
图1 合成硅酸镍的TGA-DSC曲线
Fig.1 TGA-DSC results of NiSiO3 under controlled condition in air at heating rate of 5 ℃/min
表1 样品中镍含量随温度的变化
Table 1 Content of nickel changed with temperature
1.2 实验方法
首先移取150 mL新配置的一定浓度的NH3·H2O和(NH4)2SO4混合溶液到250 mL锥形瓶中,然后加入一定质量的NiSiO3样品,立即盖上涂有凡士林的磨砂塞,以避免NH3的挥发对试验的影响,最后将锥形瓶至于恒温振荡水浴中,在设定温度下反应。同时配制5个相同条件的实验,每隔5 d取出1个,液固离心分离,分析溶液中的Ni(Ⅱ)浓度。若相邻两次取出的样品中Ni(II)浓度测量结果差别小于0.1%时,即可认为体系已达平衡。
1.3 分析与表征
根据镍精矿化学分析方法镍量的测定YS/T 341.1—2006和有色冶金分析手册中的EDTA直接滴定法测定Ni(Ⅱ)浓度;采用日本理学公司的X线衍射仪(RIGAKU-TTRⅢ)表征样品的晶体结构,计算晶粒尺寸、微观应变等,测试条件为:工作电压40 kV,工作电流250 mA,Cu Kα,扫描速度为1 (°)/min,步长0.01;采用日本JSM-6360LV 型扫描电镜(SEM)观察样品形貌;采用Universal公司生产的SDTQ600V8.0 Build95型差热-热重分析仪,在空气气氛下,对样品的热特性进行分析,升温速度5 ℃/min。
2 结果与讨论
根据Matas等[19]对橄榄石型硅酸盐矿物的热力学数据及Wang等[20]对矿物水溶液溶解平衡中提供的热力学数据,计算了硅酸镍在NH3-(NH4)2SO4-H2O体系中的热力学,在25 ℃时,反应的吉布斯自由能DGfQ=-6.869 kJ/mol,随温度升高反应越难进行。
将合成的硅酸镍分别在400,500,600,700,800,900和1 000 ℃下煅烧2 h,所得样品的XRD图谱如图2所示。
随着温度的升高,硅酸镍颜色由淡绿色→灰色→黑色→深绿色变化。由图2可知,合成的硅酸镍在煅烧温度为700 ℃时为非晶状态。随着温度的升高,衍射峰的强度不断增加,其结晶性能变好。800 ℃时出现结晶的NiSiO3和SiO2的衍射峰,900 ℃时衍射峰变得尖锐,NiSiO3的所有特征峰均显现。出现SiO2特征峰的原因是硅酸钠过量,水解形成硅酸胶体,这也是热重曲线上有质量损失的原因之一。
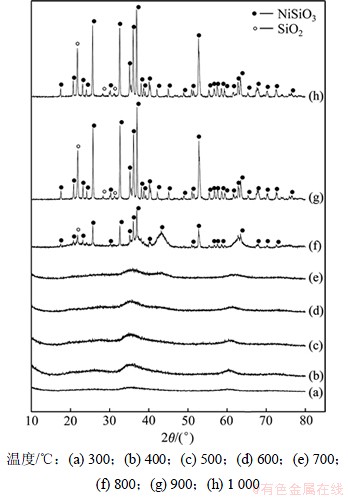
图2 不同温度下获得的样品的XRD图谱
Fig.2 XRD patterns of NiSiO3 obtained at different temperatures
将样品的XRD图谱采用Jade 6.0进行计算与拟合,可得出不同温度下样品的结晶度,如图3所示。
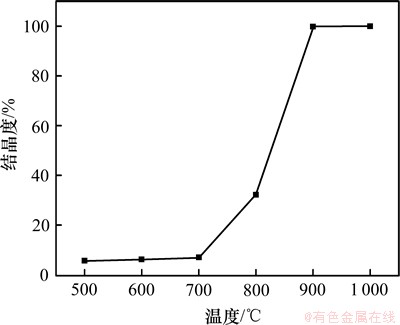
图3 结晶度与煅烧温度关系
Fig.3 Relationship between crystallinity and temperature
由图3可知,NiSiO3在700 ℃以下时为非晶状态,无法计算结晶度。随着煅烧温度的升高,结晶度逐渐提高,800 ℃时结晶度迅速提高到32.5%,900 ℃达到100%。
采用NiSiO3的(020)晶面计算其晶粒尺寸,(112)晶面计算其微观应变,晶粒粒径和微观应变随煅烧温度的变化见表2。
表2 不同温度下样品的晶粒粒径及微观应变
Table 2 Crystallite size and strain at different temperature
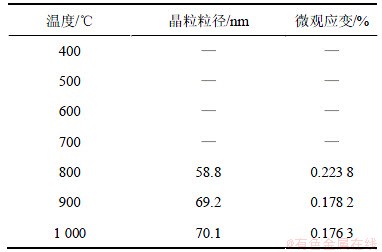
由于不能对非晶状态的物质的图谱进行拟合,因此不能获得700 ℃以下样品的晶粒粒径和微观应变,随着煅烧温度的升高,晶粒尺寸逐渐增大,微观应变逐渐减小,但变化不是很大。
图4所示为不同煅烧温度下样品的SEM图。结合图2与图4可知,非晶态或结晶度不高的NiSiO3表面粗糙,为絮状颗粒的团聚体,当转变为结晶态的NiSiO3时,絮状颗粒消失,颗粒表面光滑,有明显的烧结颈。
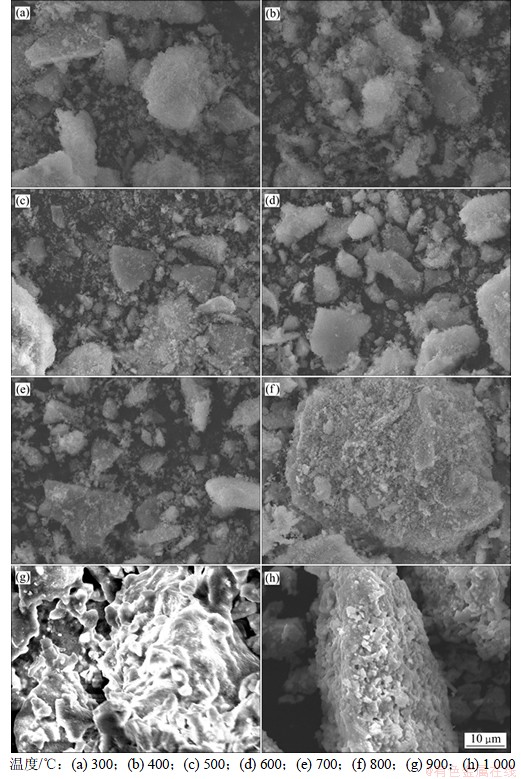
图4 不同温度下获得的NiSiO 3 SEM照片
Fig.4 SEM images of sample prepared at different temperatures
煅烧温度不同使得样品的晶体结构存在差异,其外观、颗粒的形貌、结晶度、晶粒尺寸、微观应变均不同,从而影响样品的性能。在总氨浓度3.5 mol/L,n(NH3)/n(NH4+)为2:3,温度为25 ℃ 条件下,研究不同煅烧温度下获得NiSiO3的溶解平衡,结果见图5。
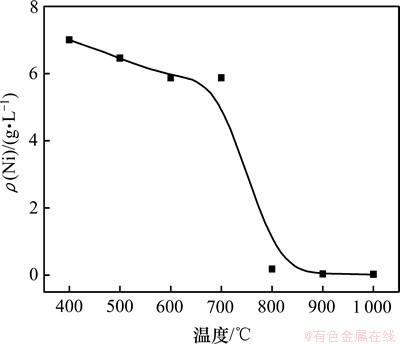
图5 煅烧温度与NiSiO3溶解平衡浓度关系
Fig.5 Relationship between temperature and solubility of NiSiO3
由图5可知,非晶态的NiSiO3在NH3-(NH3)2SO4- H2O中溶解时,Ni2+的质量浓度可达7 g/L,一旦转变为结晶的NiSiO3,溶解平衡浓度急剧下降,几乎不溶解。其原因是由于非晶态NiSiO3处于热力学的亚稳态,具有较高的自由能,具有更高的活性,溶解度更大。
3 结论
(1) 不同煅烧温度下获得的NiSiO3晶体结构不同。随着温度的升高,NiSiO3结晶度及晶粒尺寸逐渐增大、微观应变逐渐减小、晶体的结构越完整,稳定性越高,溶解度越小。
(2) 非晶态的NiSiO3在总氨浓度3.5 mol/L,n(NH3)/n(NH4+)为2:3,温度为25 ℃条件下,Ni2+质量浓度可达7 g/L,而结晶态的NiSiO3溶解平衡时,Ni2+质量浓度只有0.029 g/L。
参考文献:
[1] Agatzini-Leonardou S, Zafiratos I G, Spathis D. Beneficiation of a Greek serpentinic nickeliferous ore (Part Ⅰ). Mineral processing[J]. Hydrometallurgy, 2004, 74(3/4): 259-265.
[2] Harris C T, Peacey J G, Pickles C A. Selective sulphidation of a nickeliferous lateritic ore[J]. Minerals Engineering, 2011, 24(7): 651-660.
[3] 李光辉, 饶明军, 姜涛, 等. 红土镍矿还原焙烧-磁选制取镍铁合金原料的新工艺[J]. 中国有色金属学报, 2011, 21(12): 3137-3142.
LI Guanghui, RAO Mingjun, JIANG Tao, et al. Innovative process for preparing ferronickel materials from laterite ore by reduction roasting-magnetic separation[J]. The Chinese Journal of Nonferrous Metals, 2011, 21(12): 3137-3142.
[4] 梁威, 王晖, 符剑刚, 等. 从低品位红土镍矿中高效回收镍铁[J]. 中南大学学报: 自然科学版, 2011(8): 2173-2177.
LIANG Wei, WANG Hui, FU Jiangang, et al. High recovery of ferro-nickel from low grade nickel laterite ore[J]. Journal of Central South University: Science and Technology,2011(8): 2173-2177.
[5] 刘志宏, 马小波, 朱德庆, 等. 红土镍矿还原熔炼制备镍铁的试验研究[J]. 中南大学学报: 自然科学版, 2011(10): 2905-2910.
LIU Zhihong, MA Xiaobo, ZHU Deqing, et al. Preparation of ferronickel from laterite ore in reduction smelting process[J]. Journal of Central South University: Science and Technology,2011(8): 2173-2177.
[6] Agatzini-Leonardou S, Zafiratos I G. Beneficiation of a Greek serpentinic nickeliferous ore (Part Ⅱ): Sulphuric acid heap and agitation leaching[J]. Hydrometallurgy, 2004, 74(3/4): 267-275.
[7] Karidakis T, Agatzini-Leonardou S, Neou-Syngouna P. Removal of magnesium from nickel laterite leach liquors by chemical precipitation using calcium hydroxide and the potential use of the precipitate as a filler material[J]. Hydrometallurgy, 2005, 76(1/2): 105-114.
[8] McDonald R G, Whittington B I. Atmospheric acid leaching of nickel laterites review (Part Ⅱ): Chloride and bio- technologies[J]. Hydrometallurgy, 2008, 91(1/2/3/4): 56-69.
[9] McDonald R G, Whittington B I. Atmospheric acid leaching of nickel laterites review (Part Ⅰ): Sulphuric acid technologies[J]. Hydrometallurgy, 2008, 91(1/2/3/4): 35-55.
[10] Agatzini-Leonardou S, Tsakiridis P E, Oustadakis P, et al. Hydrometallurgical process for the separation and recovery of nickel from sulphate heap leach liquor of nickeliferrous laterite ores[J]. Minerals Engineering, 2009, 22(14): 1181-1192.
[11] LIU Wanrong, LI Xinhai, HU Qiyang, et al. Pretreatment study on chloridizing segregation and magnetic separation of low-grade nickel laterites[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(Suppl 1): s82-s86.
[12] Valix M, Cheung W H. Effect of sulfur on the mineral phases of laterite ores at high temperature reduction[J]. Minerals Engineering, 2002, 15(7): 523-530.
[13] Valix M, Cheung W H. Study of phase transformation of laterite ores at high temperature[J]. Minerals Engineering, 2002, 15(8): 607-612.
[14] FAN Chuanlin, ZHAI Xiujing, FU Yan, et al. Kinetics of selective chlorination of pre-reduced limonitic nickel laterite using hydrogen chloride[J]. Minerals Engineering, 2011, 24(9): 1016-1021.
[15] LIU Wanrong, LI Xinhai, HU Qiyang, et al. Pretreatment study on chloridizing segregation and magnetic separation of low-grade nickel laterites[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(S1): S82-S86.
[16] FAN Chuanlin, ZHAI Xiujing, FU Yan, et al. Extraction of nickel and cobalt from reduced limonitic laterite using a selective chlorination-water leaching process[J]. Hydrometallurgy, 2010, 105(1/2): 191-194.
[17] ZHANG Zebiao, WANG Wankun, PENG Jinhui. Innovative process of leaching of nickel supported activated carbon in ammonium sulfate[J]. Advanced Manufacturing Systems, 2011, 201/202/203: 1774-1779.
[18] Zuniga M, Parada L F, Asselin E. Leaching of a limonitic laterite in ammoniacal solutions with metallic iron[J]. Hydrometallurgy, 2010, 104(2): 260-267.
[19] Matas J, Ricard Y, Lemelle L, et al. An improved thermodynamic model of metal-olivine-pyroxene stability domains[J]. Contributions to Mineralogy and Petrology, 2000, 140(1): 73-83.
[20] WANG Yifeng, XU Huifang. Prediction of trace metal partitioning between minerals and aqueous solutions: A linear free energy correlation approach[J]. Geochimica Et Cosmochimica Acta, 2001, 65(10): 1529-1543.
(编辑 赵俊)
收稿日期:2012-06-25;修回日期:2012-09-05
基金项目:国家重点基础研究发展计划(“973”计划)项目(2007CB613604);教育部新世纪优秀人才资助计划(NCET-11-0517)
通信作者:李启厚(1973-),男,湖北仙桃人,教授,从事冶金复杂物料处理及功能粉体材料制备;电话:0731-88830478;E-mail: csuliuzhiyong@163.com