DOI: 10.11817/j.issn.1672-7207.2018.09.003
氯化胆碱-乙二醇低共熔溶剂中Sn2+/Sn的电化学行为
苏波1,李坚1, 2,华一新1, 2,徐存英1, 2,李艳1, 2,艾刚华1
(1. 昆明理工大学 冶金与能源工程学院,云南 昆明,650093;
2. 省部共建复杂有色金属资源清洁利用国家重点实验室,云南 昆明,650093)
摘要:采用循环伏安法和计时安培法研究在氯化胆碱-乙二醇低共熔溶剂SnCl2·2H2O/[1ChCl:2EG] DES中,Sn2+/Sn的电化学行为和Sn的电结晶形核机理。研究结果表明:Sn的电沉积需要一定的过电位,并随温度的升高,所需的过电位减小;获得323 K时的传递系数α为0.35,Sn2+的扩散系数为1.628×10-6cm2/s;在303~343 K内,Sn2+在SnCl2·2H2O/[1ChCl:2EG] DES中的电还原反应属于受扩散控制的准可逆过程;当阴极施加电位较小(较正)时,Sn在玻碳电极上的电结晶过程为受扩散控制的三维瞬时形核,当阴极施加电位较大(较负)时,Sn的电结晶过程更趋向于三维连续形核。
关键词:低共熔溶剂;氯化胆碱-乙二醇;电化学;电结晶;形核机理
中图分类号:TF111.52 文献标志码:A 文章编号:1672-7207(2018)09-2129-08
Electrochemistry of Sn2+/Sn in choline chloride-glycol deep eutectic solvents
SU Bo1, LI Jian1, 2, HUA Yixin1, 2, XU Cunying1, 2, LI Yan1, 2, AI Ganghua1
(1. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China;
2. State Key Laboratory of Complex Nonferrous Metal Resources Cleaning Utilization, Kunming 650093, China)
Abstract: The electrochemical behaviors of Sn2+/Sn and the nucleation mechanism of the electrocrystallization of tin in choline chloride-glycol deep eutectic solvents SnCl2·2H2O/[1ChCl:2EG] DES were studied by means of cyclic voltammetry and chronoamperometry. The results show that a certain overpotential is required for tin electrodeposition and the required overpotential decreases with the increase of temperature. The transfer coefficient α is calculated to be 0.35 and the diffusion coefficient of Sn2+ is estimated to be 1.628×10-6cm2/s at 323 K. The electroreduction of Sn2+ in SnCl2 ·2H2O/[1ChCl:2EG] DES is a quasi-reversible process controlled by diffusion at 303-343 K. When the applied potential on cathode is lower(more positive), the initial electrocrystallization process of Sn on a GC electrode is found to be a three-dimensional instantaneous nucleation under diffusion control by chronoamperometry. When the applied potential on cathode is larger(more negative), it is more likely to be three-dimensional progressive nucleation.
Key words: deep eutectic solvent; choline chloride-ethylene glycol; electrochemistry; electrocrystallization; nucleation mechanism
对于许多金属(如铜、铅、镍、锡、金、银等)的提纯,电解精炼是一种十分有效而被广泛采用的方法。金属锡因具有无毒性、良好的韧性以及耐腐蚀性等优点,被广泛应用于电子通信、食品包装、化工、建筑、机械、原子能及航天工业等领域[1]。火法冶炼产出的粗锡中常含有铁、铜、砷、锑、铅、铋和银等杂质,可通过火法精炼或电解精炼加以脱除至允许的限度。粗锡电解精炼主要采用酸性电解液,有硫酸亚锡-硫酸-苯酚磺酸(或甲酚磺酸)、硅氟酸亚锡-硅氟酸-硫酸等电解液体系,电解液具有较强的腐蚀性,且电解的电流效率也因阴极析氢等副反应往往低于90%。离子液体(ionic liquid solvents,ILs)/低共熔溶剂(deep eutectic solvents,DESs)不仅具有较宽的电化学窗口,而且低毒可降解、不易挥发、热稳定性较高[2],低共熔溶剂的物理化学性质与离子液体极为相似,因此,其被归为一类新型离子液体或类离子液体[3-4],一般由氯化胆碱与具有氢键供体的化合物(如尿素、乙二醇、丙三醇及草酸等)混合共熔而成[5]。基于氯化胆碱的低共熔溶剂作为电解质,已成功用于不同基底上电沉积多种金属和合金[6],不仅可以减少析氢带来的影响[7],而且可电沉积出如轻金属、难熔金属以及化合物半导体[8],因而被视为一类新型的绿色溶剂。本文作者以含有氯化亚锡的氯化胆碱-乙二醇低共熔溶剂(SnCl2·2H2O/[1ChCl:2EG] DES)为电解液,对Sn-Pb合金(含Pb 5%~20%)进行电解分离,获得树枝状锡粉(Sn质量分数w(Sn)≥99.95%)。通过循环伏安法、计时安培法等电化学方法,对Sn2+在阴极的电还原形核机理进行相关研究和讨论。
1 实验部分
1.1 试剂与材料
氯化胆碱(HOC2H4N(CH3)3Cl,简写为ChCl,w(ChCl)≥98%,AR,国药化学试剂公司);乙二醇(HOC2H4OH,简写为EG,w(EG)≥98%,AR,西陇化工股份有限公司);二水合氯化亚锡(SnCl2·2H2O,w(SnCl2·2H2O)≥98.5%,AR,成都科龙化工试剂厂);无水乙醇(AR,天津市风船化学试剂科技有限公司);α-氧化铝抛光粉(w(Al2O3)>99%,天津艾达恒晟科技发展有限公司)。
1.2 实验设备与分析仪器
RET basic恒温磁力加热搅拌器(德国IKA公司);DZF-6090真空干燥箱(上海精宏实验设备有限公司);DU-20型电热油浴锅(上海一恒科技有限公司);CHI760D电化学工作站(上海辰华仪器有限公司);FEI Quanta 200x扫描电子显微镜(荷兰PEI公司)。
1.3 实验方法
低共熔溶剂的制备:由于氯化胆碱具有较强的吸水性,实验前先将氯化胆碱置于真空干燥箱中,于80 ℃干燥24 h,之后按照物质的量比为1:2分别称取一定质量的氯化胆碱和乙二醇置于锥形瓶中适当混合,再于70 ℃的油浴锅中加热搅拌直到变为无色清亮的液体即为氯化胆碱-乙二醇低共熔溶剂(1ChCl:2EG DES)。将所得的低共熔溶剂密封,于70 ℃真空干燥箱中保存备用;称取一定质量的SnCl2·2H2O,加入已制备好的1ChCl:2EG DES中,于70 ℃油浴锅内充分搅拌、溶解形成均质液体,得到含有一定浓度SnCl2·2H2O的1ChCl:2EG DES。
电化学测试:采用三电极体系,以玻碳(GC)电极(直径4 mm)为工作电极,铂圆盘电极(直径4 mm)为对电极,银丝电极(直径1 mm)为准参比电极,分别在不同扫速(20~100 mV/s)和不同温度(303~343 K)下进行循环伏安(CV)测试;采用恒电位阶跃法进行计时电流测试。电极在每次测量前先用金相砂纸打磨,然后用粒度为0.5 μm的Al2O3抛光粉打磨至镜面光亮以防止记忆效应,然后,用无水乙醇和蒸馏水冲洗干燥后备用。
电沉积实验:石墨片作阳极(长×宽×厚为40 mm×20 mm×2 mm),纯铜片作阴极(长×宽×厚为35 mm×20 mm×0.5 mm),阴阳极间距为20 mm。电极在进行实验前先进行磨光、抛光处理,再经无水乙醇浸泡超声去脂后用去离子水冲洗,冷风吹干后备用。
2 结果与讨论
2.1 扫速对SnCl2·2H2O/[1ChCl:2EG]DES循环伏安的影响
采用不同的电势扫描速度对0.1 mol/L SnCl2·2H2O/[1ChCl:2EG] DES进行循环伏安测试,所得结果如图1所示,相关数据如表1所示。
从图1可以看出:随着扫速的增加,阴、阳极的峰值电流密度ipc与ipa随之增大。同时,阴极峰值电势Epc和半峰电势Epc/2发生较明显的负偏移,而阳极峰值电势则发生正偏移;此外,阴极峰值电流密度ipc与扫速的平方根v1/2呈良好的线性关系,表明电极反应受扩散控制。由表1可知:在相同扫速条件下,ipc/ipa≠1,且电势差△Ep=|Epc-Epa|远大于可逆过程的标准值(2.3RT/(nF)=32 mV,323 K时);|Epc-Epc/2|随扫速增大而明显增大,即使在最低扫速下,其值也远大于可逆过程的标准值(2.20RT/(nF)=30.6 mV,323 K)。以上结果表明,SnCl2·2H2O/ [1ChCl:2EG] DES中Sn2+的电化学还原过程是受扩散控制的准可逆过程[9]。
表1 0.1 mol/L SnCl2·2H2O/[1ChCl:2EG] DES在不同电势扫速下循环伏安曲线的相关数据(323 K)
Table 1 Values related to CV at different scan rates in 0.1 mol/L SnCl2·2H2O/[1ChCl:2EG] DES at 323 K


图1 扫速对0.1 mol/L SnCl2·2H2O/[1ChCl:2EG] DES循环伏安的影响(GC电极,323 K)
Fig. 1 Effect of scan rate on cyclic voltammograms in 0.1 mol/L SnCl2·2H2O/[1ChCl:2EG] DES (GC electrode, 323 K)
不可逆体系中电极反应的阴极峰值电流与对应的扫速平方根v1/2的关系同样适用于准可逆体系,可用Randles-Sevick方程表示如下[9]:
 (1)
(1)
式中:ipc为阴极峰值电流密度,mA/cm2;n为交换电子数;F为法拉第常数,96 484 C/mol;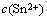 为Sn2+的浓度,mol/L;
为Sn2+的浓度,mol/L;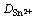 为Sn2+的扩散系数,cm2/s;α为传递系数;nα为速率决定步骤中的电子转移数;v为扫速,V/s;R为气体常数,8.314 kJ/mol;T为热力学温度,K。
为Sn2+的扩散系数,cm2/s;α为传递系数;nα为速率决定步骤中的电子转移数;v为扫速,V/s;R为气体常数,8.314 kJ/mol;T为热力学温度,K。 可由式(2)[9]得到
可由式(2)[9]得到
 (2)
(2)
根据式(2)和表1的相关数据可以求得温度为323 K时的传递系数 为0.35。
为0.35。
2.2 温度对扩散系数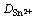 的影响
的影响
对0.1 mol/L SnCl2·2H2O/[1ChCl:2EG] DES于不同温度下测试的循环伏安曲线如图2所示。
为了研究温度对阴极峰值电流密度ipc和峰电势Epc的影响,将图2所示曲线的相关动力学参数列于表2。

图2 温度对0.1 mol/L SnCl2·2H2O/[1ChCl:2EG] DES的循环伏安的影响(GC电极,扫速20 mV/s)
Fig. 2 Effect of temperature on cyclic voltammograms in 0.1 mol/L SnCl2·2H2O/[1ChCl:2EG] DES (GC electrode, 20 mV/s)
表2 0.1 mol/L SnCl2·2H2O/[1ChCl:2EG] DES在不同温度下循环伏安曲线的相关数据(20 mV/s)
Table 2 Values related to CV at different temperatures in 0.1 mol/L SnCl2·2H2O/[1ChCl:2EG] DES at 20 mV/s
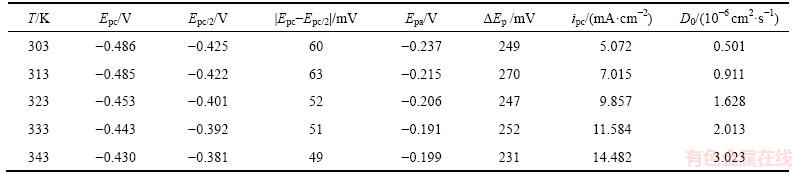
由表2可知:随着温度升高,阴极峰电势Epc发生正向偏移,同时阴极峰值电流密度ipc也明显增大,表明温度升高可加速金属锡的电沉积。由图2的局部放大图可见:在阴极和阳极扫描之间的阴极电流处出现形核电流环,表明阴极形核需要一定的过电位,并随温度的升高,所需的形核过电位有所减小。这主要是因为在较高温度下,阴极还原反应所需的驱动力减小。随温度升高,阳极峰电流密度ipa也明显增大,阳极峰电势Epa也发生正向偏移。△Ep=|Epc-Epa| (284~315 mV)远大于可逆过程的标准值2.3RT/(nF)[9],再次表明在SnCl2·2H2O/[1ChCl:2EG] DES中,Sn2+的还原属于准可逆过程。
将不同温度下的阴极峰电流密度ipc对扫速平方根作图,如图3所示。

图3 不同温度下阴极峰电流密度(ipc)与扫速平方根(v1/2)的关系
Fig. 3 Plot of cathodic peak ipc as a function of square root of scan rate v1/2 at different temperatures
由式(1)和图3求得不同温度下Sn2+的扩散系数见表2。由表2可以看出:随着温度升高,Sn2+离子的扩散系数明显增大。因为温度升高使SnCl2·2H2O/ [1ChCl:2EG] DES的黏度降低,从而使Sn2+迁移速率明显增大[10-11]。将不同温度的扩散系数的对数 对温度的倒数T-1作图,如图4所示。
对温度的倒数T-1作图,如图4所示。

图4 扩散系数的对数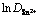 与温度倒数T-1的关系
与温度倒数T-1的关系
Fig. 4 Plot of logarithm of diffusion coefficient against reciprocal value of absolute temperature T-1
against reciprocal value of absolute temperature T-1
从图4可以看出: 与T-1呈良好的线性关系,符合阿伦尼乌斯公式[12]:
与T-1呈良好的线性关系,符合阿伦尼乌斯公式[12]:
 (3)
(3)
由式(3)可以得到Sn2+的离子扩散表观活化能Ea=39.69 kJ/mol。在本文测试条件下和0.1 mol/L SnCl2·2H2O/[1ChCl:2EG] DES中,温度从303 K升高至343 K,Sn2+的扩散系数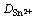 从0.501×10-6 cm2/s逐渐增大至3.023×10-6 cm2/s。在323 K时,Sn2+在1ChCl:2EG DES中的扩散系数为1.628×10-6 cm2/s,与相同温度下Sn2+在1ChCl:2PG DES[13]中的扩散系数(1.2×10-6 cm2/s)比较相近,但比Sn2+在1ChCl:2urea DES中的扩散系数(4.1×10-7 cm2/s)[13]大约4倍,比Sn2+在1-丁基-1-甲基吡咯烷鎓双(三氟甲磺酰)亚胺盐(BMPTFSI) ((8.6±0.4)×10-8 cm2/s)[14]中的要大得多,这一结果与离子液体的黏度差别以及Sn2+在其中的配位情况有关[15]。
从0.501×10-6 cm2/s逐渐增大至3.023×10-6 cm2/s。在323 K时,Sn2+在1ChCl:2EG DES中的扩散系数为1.628×10-6 cm2/s,与相同温度下Sn2+在1ChCl:2PG DES[13]中的扩散系数(1.2×10-6 cm2/s)比较相近,但比Sn2+在1ChCl:2urea DES中的扩散系数(4.1×10-7 cm2/s)[13]大约4倍,比Sn2+在1-丁基-1-甲基吡咯烷鎓双(三氟甲磺酰)亚胺盐(BMPTFSI) ((8.6±0.4)×10-8 cm2/s)[14]中的要大得多,这一结果与离子液体的黏度差别以及Sn2+在其中的配位情况有关[15]。
2.3 计时安培分析及金属锡电沉积形核生长机理
由前述的循环伏安曲线可知,Sn2+在GC电极上的沉积需要一定的过电位作为驱动力。为了进一步揭示金属Sn在GC电极上的形核和生长机理,采用恒电位阶跃法测定323 K下,SnCl2·2H2O/[1ChCl:2EG] DES中金属Sn电沉积初期的时间-电流暂态曲线,如图5所示。
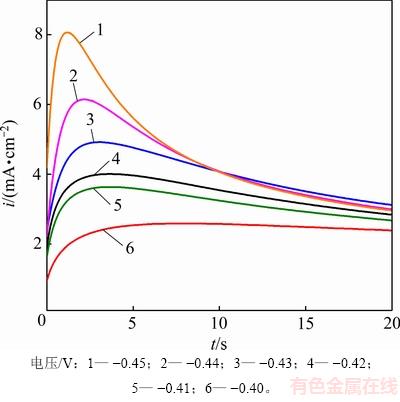
图5 SnCl2·2H2O/[1ChCl:2EG] DES中Sn电沉积的时间-电流暂态曲线(323 K)
Fig. 5 Time-current transient curves of Sn electrodeposition in SnCl2·2H2O/[1ChCl:2EG] DES (323 K)
由图5可见时间-电流暂态曲线呈现出经典的倒“V”形变化趋势,其计时电流曲线的主要特征为:在电沉积初期极短时间内,电流密度急剧增大。这是因为在电结晶初期,随着晶核数量的增加以及新相的生成,电活性区域的面积不断增加,导致电流密度迅速升高并达到最大值im;此后,随着扩散层厚度的增加,电极表面的电活性物质开始贫化,电流密度缓慢衰减,最终趋于平稳,Sn的电结晶过程经历了生长中心的消失和再生长的交替过程,这表明Sn在电沉积的初期为受扩散控制的三维异相形核过程[16]。研究还发现:随着施加在电极表面的电位增加,电流密度最大值im也增大,达到最大电流密度所需的时间tm反而缩短,说明Sn的形核过程并未遵循理想的科雷特尔方程[17]。目前,2种极端的形核机理分别归为“瞬时形核模型”和“连续形核模型”,其中“瞬时形核模型”是指在电位阶跃一开始,所有的活性位点都被激活,而“连续形核模型”则是随着时间的推移,活性位点被逐渐激活的模型。为了区分2种形核模式,SCHARIFKER等[18]将计时安培分析实验结果与理论无因次曲线((i/im)2与t/tm的关系曲线)所对应结果进行比较,进一步修正拟合得到三维瞬时形核模型和三维连续形核模型的理论表达式,公式如下。
三维瞬时形核:
 (4)
(4)
三维连续形核:
 (5)
(5)
利用图5中的数据,以(i/im)2 对t/tm作图,并与理论的三维形核生长模型曲线进行对比,结果如图6所示。
由图6可见:金属Sn在GC电极上的形核初期,大部分实验数据点都落在了2种理论形核模型曲线之间;当电极表面施加的电位较小(-0.40~-0.43 V)时,实验结果与三维瞬时形核模型所得结果比较吻合;而当电极表面施加的电位较大(-0.44~-0.45 V),t/tm<1时,实验结果接近三维瞬时形核模型,但当t/tm>1时,实验结果逐渐偏离三维瞬时形核模型曲线所得结果;转而偏向三维连续形核模型曲线。这说明在电极表面施加较负的电位时,随电沉积时间的延长,电极表面的瞬时活性位点被大量占据,开始出现有限数量的三维连续形核活性位点。

图6 时间-电流实验数据量纲一曲线与理论三维瞬时/连续形核模型曲线的对比
Fig. 6 Comparison of dimensionless experimental data dimensional normalization derived from current time-transients with theoretical models for 3D instantaneous and progressive nucleation
根据ABYANEH等[19]的推导,可由暂态电流最大值求得基体表面法向生长速率常数 ,计算式如下:
,计算式如下:
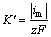 (6)
(6)
式中: 为法向生长速率常数,mol/(cm2·s);im为暂态曲线峰值电流密度,mA/cm2;z为电荷转移数;F为法拉第常数,C/mol。
为法向生长速率常数,mol/(cm2·s);im为暂态曲线峰值电流密度,mA/cm2;z为电荷转移数;F为法拉第常数,C/mol。
将时间电流曲线中不同阶跃电位下的峰值电流代入式(6)求得 ,将
,将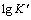 对阶跃电位E作图,如图7所示。
对阶跃电位E作图,如图7所示。
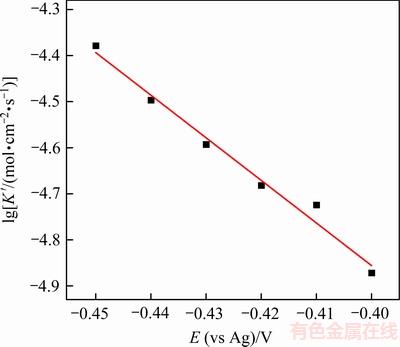
图7 Sn晶体生长速率的对数与外加阶跃电位的关系
Fig. 7 Plot of logarithm of Sn crystal growth rate as a function of step potential
由图7可知: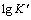 与E呈现良好的线性关系。晶体的生长速率随阶跃电位的负移而增大,说明外加电位作为驱动力,对晶体生长具有显著的促进作用。进一步根据下式[18, 20]可求得晶核数密度N:
与E呈现良好的线性关系。晶体的生长速率随阶跃电位的负移而增大,说明外加电位作为驱动力,对晶体生长具有显著的促进作用。进一步根据下式[18, 20]可求得晶核数密度N:

 (7)
(7)
式中:i为电流密度,mA/cm2;M为沉积相的摩尔质量,kg/mol; 为沉积相的密度,kg/m3。
为沉积相的密度,kg/m3。
将 对阶跃电位E作图,如图8所示。
对阶跃电位E作图,如图8所示。

图8 Sn晶核数密度与外加阶跃电位的关系
Fig. 8 Plot of logarithm of number density of Sn nucleus N as a function of step potential
由图8可知:晶核数密度 随阶跃电位的负移呈线性增加,但由于晶体表面的活性位点是有限的,虽然外加电位的增大会引起活性位点数目的增加,但在有限的区域范围内活性位点数目增加有限,随着时间的推移,基体表面可以利用的位点将被逐渐占据,因此,当外加电位增大到一定程度后,晶核数密度将不再发生明显变化。
随阶跃电位的负移呈线性增加,但由于晶体表面的活性位点是有限的,虽然外加电位的增大会引起活性位点数目的增加,但在有限的区域范围内活性位点数目增加有限,随着时间的推移,基体表面可以利用的位点将被逐渐占据,因此,当外加电位增大到一定程度后,晶核数密度将不再发生明显变化。
以纯铜片为基体,分别施加-0.43 V和-0.45 V电位进行电沉积60 s,对阴极沉积物表面进行SEM分析,结果如图9所示。从图9可见:外加电位对晶核数密度和晶粒大小有较明显的影响。
当外加电位为-0.43V时(图9(a)),Sn晶粒分布均匀,形貌似略带棱角的小球,晶粒直径集中在1 μm左右,但基体表面仍有大量未被占据的位点;当外加电位负移至-0.45 V时(图9(b))时,大量位点被激活,晶核数密度显著增加,同时晶粒直径有所减小,这与利用时间-电流曲线计算的结果一致。这表明电位负移,Sn2+发生电还原的驱动力增加,使基体表面的形核速度加快,活性位点几乎全部被占据;随着时间的延长,相邻晶核必将长大、靠拢和交叠,从而出现团聚现象[21]。在本文的实验条件下,铜基体表面相较GC电极的镜面而言较粗糙,在极化开始的瞬间活性位点更易转化为晶核,在初期的形核成长过程中得到的锡晶粒呈现类似球状均匀生长的现象,这与瞬时形核机理相符。

图9 不同外加电位下Sn在铜基体上电沉积早期形貌的SEM图
Fig. 9 SEM micrograph of Sn deposits initial stage on Cu substrate at different potentials
3 结论
1) 循环伏安曲线出现1对明显的氧化还原峰,说明Sn2+/Sn在GC电极表面发生电化学还原/氧化过程,同时表明1ChCl:2EG DES可作为电解质用于Sn的电解沉积和粗锡的电解精炼。
2) 在303~343 K内,SnCl2·2H2O/[1ChCl:2EG] DES中Sn2+的电化还原反应属于受扩散控制的准可逆过程,Sn2+的扩散系数为(0.501~3.023)×10-6 cm2/s,并随溶液温度的升高逐渐增大。
3) 当施加在电极表面的电位较小时,金属Sn的形核属于三维瞬时形核;而当施加电位增大时,形核过程逐渐过渡到更符合三维连续形核,特别是当沉积时间延长时更符合这一规律。
参考文献:
[1] SEKAR R, EAGAMMAI C, JAYAKRISHNAN S. Effect of additives on electrodeposition of tin and its structural and corrosion behaviour[J]. Journal of Applied Electrochemistry, 2010, 17(1): 87-97.
[2] 邓友全. 离子液体: 性质、制备和应用[M]. 北京: 中国石化出版社, 2006: 377-384.
DENG Youquan. Ionic liquids: properties, preparation and applications[M]. Beijing: Sinopec Press, 2006: 377-384.
[3] ABBOTT A P, FRISCH G, HARTLEY J, et al.Processing of metals and metal oxides using ionic liquids[J]. Green Chemistry, 2011, 13(3): 471-481.
[4] ABBOTT A P, CAPPER G, DAVIES D L, et al. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains electronic supplementary information (ESI) available: plot of conductivity vs. temperature for the ionic liquid formed from zinc chloride and choline chloride (2:1)[J]. Chemical Communications, 2001, 19: 2010-2011.
[5] ABBOTT A P, CAPPER G, MCKENZIE K J, et al. Electrodeposition of zinc-tin alloys from deep eutectic solvents based on choline chloride[J]. Journal of Electroanalytical Chemistry, 2007, 599(2): 288-294.
[6] ANICAI L, PETICA A, COSTOVICI S, et al. Electrodeposition of Sn and NiSn alloys coatings using choline chloride based ionic liquids: evaluation of corrosion behavior[J]. Electrochimica Acta, 2013, 114: 868-877.
[7] WHITEHEAD A H, P LZLER M, GOLLAS B. Zinc electrodeposition from a deep eutectic system containing choline chloride and ethylene glycol[J]. Journal of the Electrochemical Society, 2010, 157(6): D328-D334.
LZLER M, GOLLAS B. Zinc electrodeposition from a deep eutectic system containing choline chloride and ethylene glycol[J]. Journal of the Electrochemical Society, 2010, 157(6): D328-D334.
[8] DALE P J, SAMANTILLEKE A P, SHIVAGAN D D, et al. Synthesis of cadmium and zinc semiconductor compounds from an ionic liquid containing choline chloride and urea[J]. Thin Solid Films, 2007, 515(15): 5751-5754.
[9] BARD A J, FAULKNER L R. Electrochemical methods: fundamentals and applications[M]. New York: J Wiley & Sons, 2000: 230-232.
[10] KUZNETSOV S A, GAUNE-ESCARD M. Kinetics of electrode processes and thermodynamic properties of europium chlorides dissolved in alkali chloride melts[J]. Journal of Electroanalytical Chemistry, 2006, 595(1): 11-22.
[11] GUO Wujie, HOU Yucui, REN Shuhang, et al. Formation of deep eutectic solvents by phenols and choline chloride and their physical properties[J]. Journal of Chemical and Engineering Data, 2013, 58(4): 866-872.
[12] 华一新. 冶金过程动力学导论[M]. 北京: 冶金工业出版社, 2004: 27-29.
HUA Yixin. The dynamics of metallurgical process[M]. Beijing: Metallurgical Industry Press, 2004: 27-29.
[13] SALOM S, PEREIRA N M, FERREIRA E S, et al. Tin electrodeposition from choline chloride based solvent: influence of the hydrogen bond donors[J]. Journal of Electroanalytical Chemistry, 2013, 703(16): 80-87.
S, PEREIRA N M, FERREIRA E S, et al. Tin electrodeposition from choline chloride based solvent: influence of the hydrogen bond donors[J]. Journal of Electroanalytical Chemistry, 2013, 703(16): 80-87.
[14] TACHIKAWA N, SERIZAWA N, KATAYAMA Y, et al. Electrochemistry of Sn(Ⅱ)/Sn in a hydrophobic room- temperature ionic liquid[J]. Electrochimica Acta, 2008, 53(22): 6530-6534.
[15] YAMAGATA M, TACHIKAWA N, KATAYAMA Y, et al. Electrochemical behavior of several iron complexes in hydrophobic room-temperature ionic liquids[J]. Electrochimica Acta, 2007, 52(9): 3317-3322.
[16] ZHANG Qibo, HUA Yixin. Influence of [B-mim]HSO4 on the nucleation and growth of zinc on aluminum from acidic sulphate bath[J]. Asian Journal of Chemistry, 2013, 25(2): 701-707.
[17] RADISIC A, LONG J G, HOFFMANN P M, et al. Nucleation and growth of copper on TiN from pyrophosphate solution[J]. Journal of the Electrochemical Society, 2001, 148: C41-C46.
[18] SCHARIFKER B, HILLS G. Theoretical and experimental studies of multiple nucleation[J]. Electrochimica Acta, 1983, 28(7): 879-889.
[19] ABYANEH M Y, HENDRIKX J, VISSCHER W, et al. The electrocrystallization of zinc from alkaline media[J]. Journal of the Electrochemical Society, 1982, 129(12): 2654-2659.
[20] SUN JIE, MING Tingyun, QIAN Huixuan, et al. Electrochemical behavior of copper electrodeposition in BMIMPF6Ionic liquid[J]. Chemical Journal of Chinese Universities, 2018, 39(7): 1497-1502.
[21] NAN Tianxiang, YANG Jianguang, CHEN Bing. Electrochemical mechanism of tin membrane electrodeposition under ultrasonic waves[J]. Ultrasonics Sonochemistry, 2018, 42: 731-737.
(编辑 刘锦伟)
收稿日期:2017-09-13;修回日期:2017-11-02
基金项目(Foundation item):国家重点基础研究发展计划(973计划)项目(2014CB643404);国家自然科学基金资助项目(51504112) (Project(2014CB643404) supported by the National Basic Research Development Program (973 Program) of China; Project(51504112) supported by the National Natural Science Foundation of China)
通信作者:李坚,教授,硕士生导师,从事有色金属冶金研究;E-mail: kglj1010@163.com