文章编号:1004-0609(2008)09-1692-07
LaNi5(111)表面结构及吸氢机理的第一性原理研究
刘奕新,郑定山,张 怡,蒋 龙,黎光旭,郭 进
(广西大学 物理科学与工程技术学院,教育部有色金属材料及其加工新技术重点实验室,南宁 530004)
摘 要:采用基于密度泛函理论的第一原理赝势平面波方法,对贮氢合金LaNi5及LaNi5(111)表面的电子结构进行计算,对H原子在LaNi5(111)表面吸附模型进行构型优化。结果表明:LaNi5(111)表面驰豫结构La原子向外凸出,Ni原子向里收缩,凹凸不平的表面层增加表面原子与H原子的接触面积,表面层的有效体积约增大2.3%,有利于H原子向块体内扩散;表面层有净余的0.5个电子,有利于表面层上的电子转移到H原子上;H2分子解离成两个H原子后在LaNi5(111)表面的平衡稳定结构与氢化物LaNi5H7晶体相同位置的结构极为相似;阐述H2分子在LaNi5(111)表面的解离吸附机理,其反应活化能约为0.27 eV。
关键词:LaNi5;电子结构;吸氢机理;第一性原理
中图分类号:TG 239.7 文献标识码:A
First principle investigations on surface structure and
mechanism of hydrogen adsorption of LaNi5(111)
LIU Yi-xin, ZHENG Ding-shan, ZHANG Yi, JIANG Long, LI Guang-xu, GUO Jin
(Key Laboratory of National Education Ministry for Nonferrous Metals and Materials Processing Technology,
College of Physics Science and Technology, Guangxi University, Nanning 530004, China)
Abstract: The electronic structures of LaNi5 hydrogen storage alloy and LaNi5(111) surface with hydrogen atoms were calculated by plane wave pseudo-potential method based on density functional theory. The results show that on the relaxed surface, La atoms protrude from surface and Ni atoms cave in, which enlarges the contacting area with H atoms. The effective volume of the surface layer is increased by 2.3%, which favors H atoms to diffuse into bulk from the surface. Calculated charge population presents negative charge on the surface, and the negative charge may transfer from the surface layer to H atoms. The stable structure by geometry optimizing after H2 molecule is dissociated into two H atoms on LaNi5(111) surface presents similar structure with hydride LaNi5H7 at the same position. The possible dissociation path and the mechanism of hydrogen-adsorbed are investigated with transition state method, and the activation energy of reaction is estimated as 0.27 eV.
Key words: LaNi5; electronic structure; hydrogen adsorption mechanism; first principle
贮氢材料作为一种新型功能材料,已引起人们的普遍关注,研究贮氢材料的微观结构以及贮氢后的状态,对于了解和改善贮氢材料的性能,寻找新的贮氢材料有着重要的意义。AB5型混合稀土合金作为贮氢材料已得到广泛使用,LaNi5是AB5型化合物中较为理想的二元贮氢材料,可作为Ni/MH电池的负极材料[1],在实验和理论上其微观结构均得到较为全面的研 究[2?6]。然而由于表面实验分析技术对表层原子结构、表面弛豫与表面电荷、以及表面势能等方面的研究, 尤其是对复杂的合金化合物表面的研究,仍然缺乏充分的实验数据。因此,通过理论计算研究合金表面的原子与电子结构已成为一种重要的晶体表面研究方法。在LaNi5合金吸氢过程中,被吸入LaNi5晶体中的H是以原子状态存在的,在LaNi5表面,H2分子首先要解离成H原子后再进入晶体。
在第一原理计算中,常用的计算模型有两种,即团簇模型和超晶胞模型。团簇模型用于模拟有限原子的孤立系统,而超晶胞模型模拟的是具有一定对称性边界条件的三维周期性系统,所以超晶胞模型比团簇模型更能反应理想状态下真实的晶体系统。本文作者采用基于密度泛函理论(DFT)的第一原理赝势平面波(PW-PP)[7]方法,计算并模拟不同初始状态下H2分子解离成两个H原子后,H原子在LaNi5(111)面上的吸附过程,对H/LaNi5(111)体系的构型以及电子结构进行研究。
1 计算方法
使用CASTEP软件[8]进行计算,先对LaNi5晶胞进行几何优化,再计算其单点能。计算中,采用2×1超晶胞模型,选择基于广义梯度近似(GGA)下的PBE[9]泛函来描述交换能Vxc,平面波截止能取为300 eV,Brillouin区的k格点取为6×6×6,计算在倒易空间上进行,模型按晶体的最低对称性(P1)进行优化。迭代过程中的收敛精度为1×10?5 eV/atom,即作用在每个原子上的力不大于0.003 eV/nm,内应力不大于 0.05 GPa。计算采用赝势模型,各原子的外层电子组态分别为:La 4s24p64d104f05s25p65d16s26p0;Ni 3s23p63d84s24p0;H 1s1。
H原子在LaNi5(111)表面的结合能?E为吸附前后超晶胞总能量的差值,用公式表述为
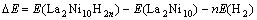
式中 E(La2Ni10H2n)为吸附后超晶胞的总能量;E(La2Ni10)为吸附前超晶胞的总能量;E(H2)为H2分子的能量。
2 计算结果与讨论
2.1 结构优化
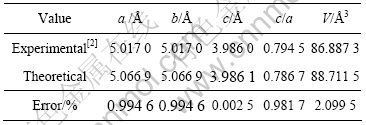
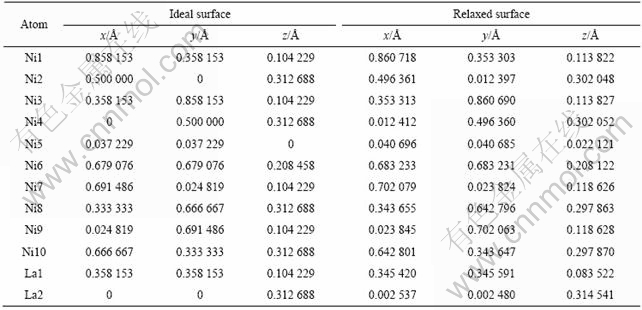
LaNi5是一种具有CaCu5型晶体结构的稀土贮氢合金,空间群为P6/mmm,其中La原子占据la位,原子坐标为(0, 0, 0),Ni原子占据两种位置:处于z=0的基平面的2c位和处于z = 1/2的中间平面的3g位,2c位原子坐标为(1/3, 2/3, 0)和(2/3, 1/3, 0),3g位原子坐标为(1/2, 0, 1/2)、(0, 1/2, 1/2) 和(1/2, 1/2, 1/2),其结构如图1所示。首先对LaNi5晶体结构模型按晶体的最低对称性(P1)进行优化,优化结果列于表1。优化后主要是a和b轴增大,而c轴变化非常小,c/a值与实验值比较接近,仅相差约0.98%,这是由于LaNi5贮氢合金存在各向异性。从表1中还可看出优化后的晶胞体积有所增加,比实验值增大约2.1%。
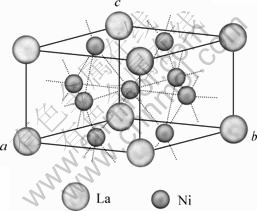
图1 LaNi5晶体模型
Fig.1 Crystal model of LaNi5
表1 LaNi5晶体晶胞常数的理论与实验值比较
Table 1 Comparison of theoretical and experimental values of lattice parameters of LaNi5 crystal
2.2 LaNi5 (111)表面的弛豫构型与电子结构
由于LaNi5(111)面密度较高,所以研究H在LaNi5(111)面上吸附时的转变过程比在其他面上更具有一般性。考虑到表面晶胞的平移对称性和局部点群对称性,以及吸附原子的边界效应,计算中采用的LaNi5(111)表面晶胞模型见图2(a)所示,晶胞表面由2×1晶胞构成,真空层厚度设为7 ?,以保证表面之间不发生明显的相互作用。
首先考虑LaNi5 (111)清洁表面的弛豫情况,对LaNi5 (111)清洁表面进行构型优化,结果列于表2。理想表面和弛豫表面的晶胞模型分别如图2(b)和图2(c)所示。从表2可以看出,最表面的La2原子向上移动凸出表面约0.001 9 nm,最表面的Ni2和Ni4原子向下移动0.010 8 nm,而Ni8与Ni10原子向下移动0.015 1 nm;次表面的Ni6原子发生很小的位置移动,仅向上移动0.000 3 nm;表面第二层La原子向下移动了 0.021 1 nm;表面第二层Ni1与Ni3原子向上移动 0.009 8 nm,Ni7与Ni9原子也向上移动约0.014 7 nm。显然,凹凸不平的表面层增加了表面原子与H原子的接触面积,有利于H原子与表面原子间的相互作用。以最表面层与第二层原子间的纵向距离来计算表面层的体积,由于La原子的上移和Ni原子的下移,导致表面晶胞的纵向体积增加约2.3%,这将有利于H原子穿过表面层而向块体内扩散。
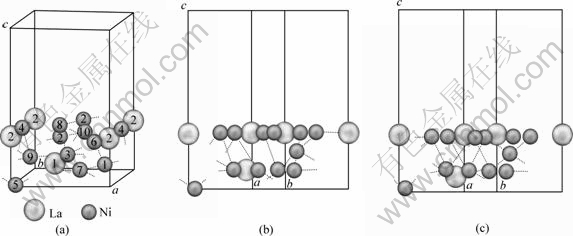
图2 LaNi5(111)表面晶胞模型
Fig.2 Crystal models of LaNi5(111) surface: (a) Surface of LaNi5(111); (b) Side view of ideal surface of LaNi5(111); (c) Side view of relaxed surface of LaNi5(111)
表2 LaNi5(111)弛豫清洁表面构型优化结果
Table 2 Geometry optimization parameters of LaNi5(111) relaxed surface
为了深入了解LaNi5(111)表面结构中原子间的相互作用,计算了理想清洁表面和弛豫清洁表面的总态密度(TDOS)及分波态密度(PDOS)图,结果如图3所示。图中将费米能级Ef处取为零点,作为能量的参考点。从图3中可以看出,理想和弛豫表面具有相似的态密度图。费米能级处理想表面和弛豫表面的态密度一样,都较低。根据结构稳定性与费米能级处的态密度关系,费米能级上的低态密度值对应于稳定结构,这是由于费米能级态密度越小,这意味着更多电子参与成键而定域,结构将更稳定[10]。LaNi5(111)晶体表面的费米面位于Ni(3d)电子能带的高能端下边沿,即态密度迅速下降部位,Ni(3d)电子能带是一个未填满能带,发生电子转移的可能性较大,说明LaNi5(111)表面仍然是金属性,与文献[7]的计算结果一样,在费米面上La的5d电子贡献很小。但与理想表面相比,弛豫表面的费米能级下方各DOS峰所处位置均向低能级方向有较小的漂移(约0.1 eV),说明结构趋于更稳定。
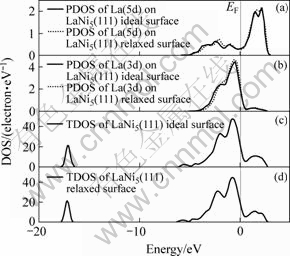
图3 LaNi5(111)理想表面及弛豫表面总态密度及分波态密度图
Fig.3 Total and partial densities of states of LaNi5(111) ideal surface and relaxed surface
LaNi5(111)表面层各原子的Mulliken电荷分析结果如表3所列。La原子失去电子,呈正电性,大部分Ni原子都得到电子,呈负电性。即表面层的原子发生了电荷转移现象,电子从La原子转移到Ni原子上。如对于La原子而言:表面第一层La2原子失去1.30个电子,第二层La1原子失去1.06个电子。而对于最表面层Ni原子:表面第一层第一类Ni原子(Ni8和Ni10)得到0.24个电子,每个第二类Ni原子(Ni2和Ni4)得到0.16个电子。表面层失去的电子比得到的电子多0.5个,即有0.5个净电子,有利于表面层的电子转移到H原子上。
表3 LaNi5(111)及LaNi5(111)—2H表面层的各原子轨道电荷分布
Table 3 Charges population on atomic orbits in LaNi5(111) and LaNi5(111)—2H surface layer

2.3 H在LaNi5 (111)表面的稳定结构及电子结构
一般认为,金属的氢化经历物理吸附、化学吸附和氢化物形成等阶段,其中物理吸附到化学吸附的过程中,H2分子裂解为H原子,进而发生化学吸附成为H+,并最终以H+的形式与合金结合成氢化物。由于这个过程经历的时间很短,难以用实验手段观察到。HAMMER等[11]成功利用能量最低反应途经研究H2分子在金属表面的吸附离解过程,结果显示H2分子通过最低能量途径直接离解到最稳定位置。
为了研究LaNi5(111)表面吸氢后的稳定位置,即形成稳定的原子吸附态,首先对H2分子离解成H原子后在LaNi5(111)表面的结构进行结构优化。H原子在LaNi5(111)表面可能的位置有如下6种:①处于表面层的Ni原子正上方的顶位T;②长桥位LB;③短桥位SB1;④短桥位SB2;⑤位于La1原子上方的空位H1;⑥位于Ni6原子上方的空位H2。结果如图4(a)所示。
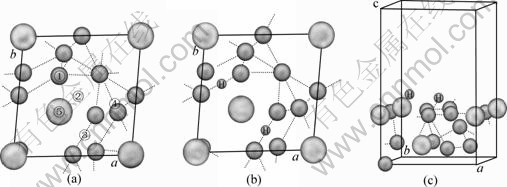
图4 LaNi5(111)及LaNi5(111)—2H晶胞模型
Fig.4 Crystal models of LaNi5(111) and LaNi5(111)—2H: (a) Top view of LaNi5(111); (b) Top view of LaNi5(111)—2H; (c) Side view of LaNi5(111)—2H
在整个结构优化过程中,保持前面的参数不变。这里没有改变La和Ni原子的坐标,是因为作用在它们上面的力很小,更重要的是H2分子离解所需时间内,相对于H原子质量较大的金属La原子和Ni原子来不及发生位置偏移。首先对两个H原子在LaNi5(111)面上的14种不同初始构型进行了结构优化,优化结果列于表4中。
表4 对LaNi5(111)面上的14种不同初始位置进行结构优化后的能量、键长及键角
Table 4 Energy, bond length and bond angle of optimized structures of 14 kinds of different initial positions of LaNi5(111) surface
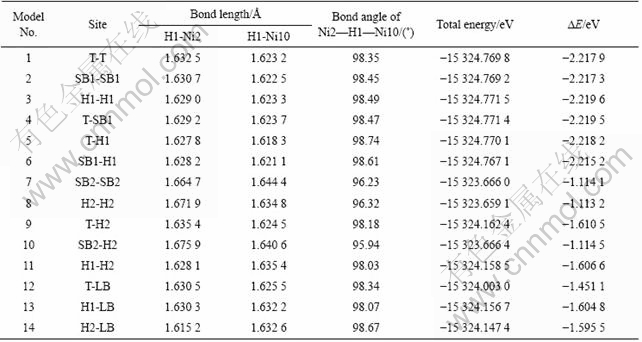
比较LaNi5(111)面上的14种不同初始位置的结构优化结果,可以发现:H原子均位于La1原子上方的空位H1表面时(Model 3),优化后的总能量最低,所得结构最为稳定。而位于Ni6原子上方的空位H2表面时(Model 8),优化后的总能量最高,即所得结构最不稳定。H原子在位于顶位T和短桥位SB1时(Model 4),优化后的结果与位于La1原子上方的空位H1表面时优化的结果非常相近;而H原子均位于短桥位SB2时(Model 7),优化后的结果与位于Ni6原子上方的空位H2表面时优化的结果非常相近。另外还发现优化后的平衡稳定结构具有对称性,如图4(b)所示,在表4中只列出H1与Ni2和Ni10键长及键角,而氢化物LaNi5H7晶体结构同一位置处的H与Ni成键的键长分别约为1.629 2 ?和1.671 3 ?,Ni—H—Ni的键角约为102.82?,由此可以看出H原子在LaNi5(111)表面的平衡稳定结构与氢化物LaNi5H7晶体相同位置的结构较为相似。H原子在进入合金形成氢化物时最优先占据在最稳定的位置,如图4(b)和4(c)所示。
图5所示为LaNi5(111)—2H平衡稳定结构(Model3)的总态密度(TDOS)及分波态密度(PDOS)图。由图5可知,位于费米能级EF下?34.1~ ?32.6 eV和?17.9~ ?16.2 eV区域内分别是La(5s)和La(5p)电子态密度的贡献。而由于氢的吸附,在?6.9~ ?3.5 eV处峰值有所增加,主要是Ni(3d)轨道电子与H的电子态密度形成的。在费米能级EF附近的峰主要由Ni(3d)轨道的电子提供。费米面位于Ni(3d)电子能带的高能端下边沿,即态密度迅速下降部位,Ni(3d)电子能带是一个未填满能带,说明LaNi5(111)—2H仍然是金属性。导带及费米能级处大部分电子来自Ni(3d)轨道,导带电子主要来自Ni(3d)和La(5d)轨道,Ni(3p)轨道电子的贡献也不可忽略。
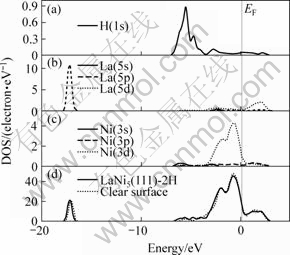
图5 LaNi5(111)—2H的总态密度及分波态密度图
Fig.5 Total and partial electron densities of states of LaNi5(111)—2H
表3也列出体系吸氢平衡后,表面金属La原子和Ni原子及吸附在表面的H原子的各原子轨道电子占据数以及净电荷数。由表3给出的原子电荷分布可见:吸附H原子后表面La2原子失去电子,Ni原子也失去电子,因而Ni—Ni键相互作用有所减弱,而每个H原子得到0.25个电子,使H 原子带有明显的负电荷。图6所示为LaNi5(111)表面及LaNi5(111)表面吸氢后的等电荷密度图。从图中可以看出,LaNi5(111)最表面层的Ni2—Ni10之间的电子云有较大的重叠,有成键作用。H原子吸附在LaNi5(111)表面后,Ni2—H及Ni10—H之间的电子云重叠较大,它们之间的相互作用要强于Ni2—Ni10原子间的相互作用,Ni2-Ni10之间的相互作用有所减弱。

图6 LaNi5(111)及LaNi5(111)—2H的等电荷密度图
Fig.6 Contour maps of electron density of LaNi5(111) and LaNi5(111)—2H: (a) LaNi5(111); (b) LaNi5(111)—2H
2.4 过渡态的能量与结构
通过几何优化方法得到H原子在LaNi5(111)表面的平衡稳定结构。为了进一步了解H2在LaNi5(111)表面的分解和反应过程,利用线性协同变换 (Linear synchronous transit–LST)与二次协同变换(Quadratic synchronous transit–QST)相结合的方法[12]及共轭梯度(Conjugate gradient–CG)方法[13?14]研究过渡态(Transition state–TS)。该方法的关键是由于对称性限制条件使得一些反应坐标(广义坐标)正好固定在过渡态上,用LST/QST方法优化不会使被优化结构离开
过渡态,优化的是其他未固定的坐标,即优化H原子的坐标,从而避免使用费时的过渡态优化标准方法。
自由H2分子距离LaNi5(111)最表面层0.4 nm处的初始位置结构,在经过构型优化后作为反应物,优化所得到的平衡稳定结构作为解离反应的产物。表5所列为反应过程中各态的构型参数及能量,从反应起点经历过渡态得到解离产物,反应路径及对应的结构如图7所示。
表5 反应历程中各中间态的构型参数与能量
Table 5 Geometry parameters and energy of each image for reaction
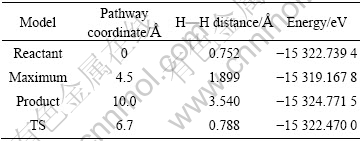
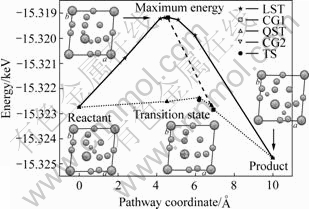
图7 搜寻过渡态时沿反应路径的能量变化曲线
Fig.7 Variation of total potential energies along reaction pathway during transition state search
从图中可以看出,在通过LST/QST方法优化得到过渡态的研究中,从反应物开始能量逐渐增加到最大值处,得到最大能量结构,此时,H—H键长约为 0.189 9 nm,H2分子已解离成H原子。但实际上这个最大能量结构可能并非我们正在搜寻的过渡态结构,随后能量又从最大值减小至生成物的能量。此后LST/QST方法继续搜寻并找到过渡态,过渡态位置如图7中所示。
根据能量变化曲线中最大能量结构与反应物之间的能量差,可求出从反应物到生成物所需克服的最大势垒约为3.57 eV,这也是H2分子在LaNi5(111)表面解离成H原子所需的最大能量。在搜寻过渡态的反应路径中,沿最低能量途径在过渡态区域(TS)出现一个势垒,即H2分子在LaNi5(111)表面的活化势垒,可由过渡态与反应物的能量之差计算得出,其值约为0.27 eV,此时H2分子间距为0.078 8 nm;越过此势垒后,H—H键距离进一步增大,此后变成原子吸附态,此活化势垒与文献[5, 15?16]实验值较为接近。解离后两个H原子在表面上等价吸附到LaNi5(111)表面,体系处于最低能量状态,形成前面所述的平衡稳定结构,此时H原子间的距离为0.354 0 nm。此过程反应的总能量即反应物与生成物的能量之差,约为?2.03 eV。在反应过程中伴随着H—H键的断裂和Ni—H键的形成,生成新键释放出的能量补偿了断键所需的部分能量,吸氢过程是放热反应,反应活化能较低。
3 结论
1) LaNi5 (111)表面驰豫结构La原子向外凸出,Ni原子向里收缩,凹凸不平的表面层增加了表面原子与H原子的接触面积,表面层的有效体积增大约2.3%,有利于H原子向块体内扩散。
2) H2分子解离成两个H原子后在LaNi5(111)表面的平衡稳定结构具有对称性,并且与氢化物LaNi5H7晶体相同位置的结构较为相似,Ni—H原子间的相互作用要强于Ni—Ni原子间的相互作用。
3) 在通过LST/ QST方法优化得到过渡态的研究中,沿最低能量途径在位能面过渡态区域出现一个位垒,即H2分子在LaNi5(111)表面活化势垒约为0.27 eV,与实验值相符,反应的能量约为?2.03 eV。
REFERENCES
[1] WILLEMS J J G, BUSCHOW K H. From permanent magnets to rechargeable hydride electrodes[J]. J Less-Common Met, 1987, 129(1/2): 13?30.
[2] MALIK S K, ARLINGHAUS F J, WALLACE W E. Calculation of the spin-polarized energy-band structure of LaNi5 and GdNi5[J]. Phys Rev B, 1982, 25(10): 6488?6491.
[3] GUO J, WEI W L, MA S Y, GAO Y J. An investigation of correlation between electronic structure of LaNi4M (M=Ni, Cu, Mn, Al) and hydrogen absorption properties[J]. Mater Sci Eng B, 2003, 98(1): 21?24.
[4] Tadaei Ito, Hideaki Ido. Electronic structures and magnetic properties of LaCo5, LaNi5, and LaCo3Ni2[J]. J Appl Phys, 2005, 97(10): 10A313.1?10A313.3.
[5] SCHLAPBACH L. XPS/UPS study of the oxidation of La and LaNi5 and of the electronic structure of LaNi5[J]. Solid State Comm, 1981, 38(2): 117?123.
[6] 陈 东, 周理海, 余本海, 王春雷, 高 涛, 张东玲. LaNi4.5Al0.5储氢合金固溶相的密度泛函研究[J]. 中国有色金属学报, 2007, 17(7): 1160?1165.
CHEN Dong, ZHOU Li-hai, YU Ben-hai, WANG Chun-lei, GAO Tao, ZHANG Dong-ling. Density functional theory study on solid solution phase of LaNi4.5Al0.5 hydrogen storage alloys[J]. The Chinese Journal of Nonferrous Metals, 2007, 17(7): 1160?1165.
[7] PAYNE M C, TETER M P, ALLAN D C. Iterative minimization techniques for ab initio total-energy calculations: molecular dynamics and conjugate gradients[J]. Rev Mod Phys, 1992, 64(4): 1045?1097.
[8] FISCHER S, KARPLUS M. Conjugate peak refinement: an algorithm for finding reaction paths and accurate transition states in systems with many degrees of freedom[J]. Chem Phys Lett, 1992, 194(3): 252?261.
[9] SEGALL M D, PHILIP J D, LINDAN M, PROBERT J. First-principles simulation: ideas, illustrations and the CASTEP code[J]. J Phys: Cond Matt, 2002, 14(11): 2717?2744.
[10] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett, 1996, 77(18): 3865?3868.
[11] LUE C S, SU T H, XIE B X, CHENG C. Comparative NMR study of hybridization effect and structural stability in D022-type NbAl3 and NbGa3[J]. Phys Rev B, 2006, 74(9): 094101.
[12] HAMMER B, JACOBSEN K W, NORSKOV J K. Dissociation Path for H2 on Al(110)[J]. Phys Rev Lett, 1992, 69(11): 1971?1974.
[13] HALGREN T A, LIPSCOMB W N. The synchronous-transit method for determining reaction pathways and locating molecular transition states[J]. Chem Phys Lett, 1977, 49(2): 225?232.
[14] BELL S, CRIGHTON J S. Locating transition states[J]. J Chem Phys, 1984, 80(6): 2464?2475.
[15] TANAKA S, CLEWLEY J D, FLANAGAN T B. Kinetics of hydrogen absorption by LaNi5[J]. J Phys Chem, 1977, 81(17): 1684?1688.
[16] OSOVIZKY A, BLOCH J, MINTZ M H, JACOB I. Kinetics of hydride formation in massive LaNi5 samples[J]. J Alloys and Comp, 1996, 245(1): 168?178.
基金项目:国家自然科学基金资助项目(50561002);广西省自然科学基金资助项目(桂科自0728028);广西大学科研重点资助项目(2004ZD04);广西教育厅科研资助项目(桂教科研[2006]26-8)
收稿日期:2008-01-10;修订日期:2008-05-05
通讯作者:郭 进,教授,博士;电话:071-3232666;E-mail: guojin@gxu.edu.cn
(编辑 龙怀中)