硫化砷渣的碱性浸出及浸出动力学
白 猛,郑雅杰,刘万宇,张传福
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘 要:采用氢氧化钠溶液浸出硫化砷渣,使As与Cu和Bi等金属有效分离,有利于硫化砷渣的综合利用。对氢氧化钠浸出硫化砷渣动力学进行探讨。研究结果表明:当反应温度为90 ℃,固液比为1?6,反应时间为1.5 h,NaOH与As2S3的摩尔比为7.2?1时,氢氧化钠浸出硫化砷渣,砷浸出率达到95.90%,铜浸出率仅为0.087%;经过氢氧化钠浸出,渣中Cu和Bi质量分数分别从10.90%和1.85%增加到50.00%和10.63%,Cu和Bi得到高度富集;溶液中As2S3与NaOH反应为收缩未反应芯扩散控制,其表观活化能为3.682 kJ/mol。
关键词:硫化砷渣;氢氧化钠;浸出;动力学
中图分类号:X705 文献标识码:A 文章编号:1672-7207(2008)02-0268-05
Alkaline leaching and leaching kinetics of arsenic sulfide residue
BAI Meng, ZHENG Ya-jie, LIU Wan-yu, ZHANG Chuan-fu
(School of Metallurgical Science and Technology, Central South University, Changsha 410083, China)
Abstract: In order to utilize arsenic sulfide residue from sulfide precipitation of contained arsenic wasted water, As was separated effectively from Cu and Bi after arsenic sulfide was leached in the sodium hydroxide solution. The leaching kinetics was studied. The results show that the leaching rate of As is up to 95.90% and the copper leaching rate is only 0.087% when the reaction temperature is 90 ℃, the ratio of solid to liquid is 1:6, the reaction time is 1.5 h, the mole ratio of NaOH to As2S3 is 7.2:1. The mass fractions of Cu and Bi in the leached slag increase from 10.90% and 1.85% to 50.00% and 10.63%, respectively. Cu and Bi are highly concentrated. The reaction between NaOH and As2S3 in the solution is controlled by diffusion and the reaction is applicable to the shrinking core model. The active energy is 3.682 kJ/mol.
Key words: arsenic sulfide residue; sodium hydroxide; leaching; kinetics
砷及其化合物严重危害人类健康,已被美国疾病控制中心和国际防癌研究机构确定为第1类致癌 物[1],主要通过饮水途径对人体造成危害人体健康[2]。因此,中国、美国、西欧、日本等把砷列为优先控制的水污染物之一。火法熔炼铜产生SO2烟气,经过洗涤后产生酸性废水含砷高达10 g/L。处理含砷废水的方法主要有萃取法、离子交换法、吸附法、石灰中和铁盐絮凝沉淀法和硫化沉淀法等[3-5],其中,萃取法、离子交换法、吸附法主要用于处理低浓度含砷废水,冶炼厂含砷废水普遍采用絮凝沉淀法和硫化沉淀法处理。采用石灰中和铁盐絮凝法处理渣量大时,需要进行固化处理,否则产生二次污染。为了利用硫化砷渣,日本住友公司通过硫酸铜置换和空气氧化法分离铜和砷,采用SO2还原滤液中砷得到As2O3。该技术成 熟,As2O3纯度高,安全性好,但工艺流程复杂,生产成本过高[6-8]。综合利用硫化砷渣具有重大意义[9-10],在此,本文作者采用氢氧化钠溶液浸出硫化砷渣,使硫化砷渣中砷与铜和铋等得到有效分离,并对浸出动力学进行研究。
1 实验原料及步骤
1.1 实验原料
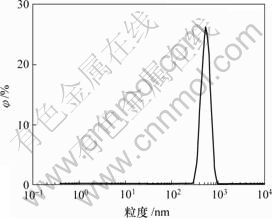
某冶炼厂含砷废水经过硫化钠沉淀处理后得到硫化砷渣,其成分如表1所示,粒度分布和形貌分别如图1和图2所示。
表1 硫化砷渣化学成分
Table 1 Chemical composition of arsenic sulfide residue w/%

φ为体积分数
图1 硫化砷粒度分布
Fig.1 Particle size distribution of arsenic sulfide residue
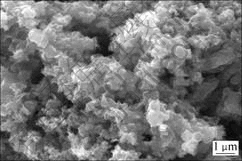
图2 硫化砷渣SEM照片
Fig.2 SEM image of arsenic sulfide residue
由表1可知,硫化砷渣中As,Cu和S质量分数分别达到18.17%,10.90%和19.25%,说明主要成分为硫化砷和硫化铜。硫化砷渣体积平均粒径为786 nm,扫描电镜(SEM)实验证实硫化砷渣由细小粒子聚集为松散体。
1.2 实 验
1.2.1 硫化砷渣的碱浸
将容积为2 L的三颈瓶置于DF-101B型集热式恒温磁力搅拌器上,或固定于超级恒温器中,按一定固液比加入氢氧化钠溶液,快速搅拌,迅速加入硫化砷渣,反应一定时间后过滤得到含砷碱浸液。
1.2.2 碱浸动力学实验
在容积为1 L带刻度的圆柱型玻璃反应器中加入氢氧化钠溶液后,迅速加入100 g硫化砷渣,JBV-III型变频调速搅拌器控制转速为400 r/min,充分搅拌。控制固液比为1?6,氢氧化钠用量为与As2S3反应的理论用量的1.2倍。在80 ℃以下通过超级恒温水浴控制反应温度,温度变化范围为-1~1 ℃;在80 ℃以上通过硅油油浴控制反应温度,温度变化范围为-2~2 ℃。在反应过程中根据反应液面补加蒸馏水,保持反应溶液体积不变。
1.2.3 硫化砷渣浸出率的测定
采用溴酸钾滴定法测定碱浸液中总砷浓度,根据测定结果计算砷浸出率(η(As))。

2 实验结果与讨论
2.1 硫化砷渣的碱性浸出
冶炼烟气废水中含有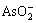 ,
,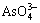 ,Cu2+,Bi3+,Pb2+,Sb3+,Zn2+和Fe2+等,加入硫化钠后发生如下 反应:
,Cu2+,Bi3+,Pb2+,Sb3+,Zn2+和Fe2+等,加入硫化钠后发生如下 反应:

将含砷废水加入硫化钠后产生的沉淀物称为硫化砷渣。实验中取300 g硫化砷渣加入NaOH溶液,当反应温度为26 ℃,固液比为1?6,反应时间为1.5 h时,NaOH与As2S3的摩尔比对砷浸出率的影响如图3所示。
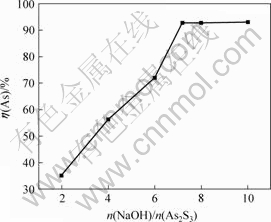
图3 NaOH与As2S3的摩尔比对砷浸出率η(As)的影响
Fig.3 Influence of mole ratios of NaOH to As2S3 on leaching rate of As
由图3可知,砷浸出率随NaOH与As2S3的摩尔比增加而增加,当NaOH与As2S3的摩尔比为7.2?1时,砷浸出率达到92.72%;继续增大NaOH用量,砷浸出率基本不变,其适宜的摩尔比为7.2?1。
硫化砷渣中加入氢氧化钠溶液,As2S3和Sb2S3沉淀发生溶解,反应如下:
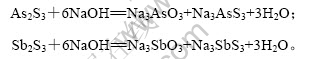
由反应原理可知,As2S3完全溶解,NaOH 与As2S3理论上的摩尔比为6?1,除其他物质消耗氢氧化钠外,实际上,只有氢氧化钠过量时,As2S3才能充分溶解,因而NaOH与As2S3实际的摩尔比大于6?1。
上述其他条件不变,当NaOH 与As2S3理论上的摩尔比为7.2?1时,反应温度对砷浸出率的影响如图4所示。
由图4可知,砷浸出率随反应温度的升高而缓慢增加;当反应温度从25 ℃增加到90 ℃时,砷浸出率从92.73%增加到96.40%。因此,反应温度对砷浸出率影响较小。
在反应温度为90 ℃,固液比为1?6,反应时间为1.5 h,NaOH与As2S3的摩尔比为7.2?1条件下,取1 kg硫化砷渣进行扩大实验,砷浸出率达到95.90%,铜 浸出率仅为0.087%。将碱浸渣进行分析,As,Cu,Bi,S,Pb,Zn,Na和Fe的质量分数分别为2.616%,50.00%,10.63%,24.42%,0.61%,0.64%,0.95%和1.22%。可见,铜和铋得到富集,砷与铜和铋分离。
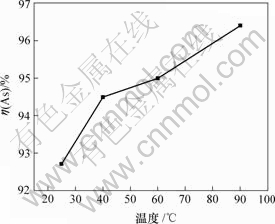
图4 反应温度对砷浸出率η(As)的影响
Fig.4 Influence of reaction temperature on leaching rate of As

1—硫化砷渣;2—碱浸渣
图5 硫化砷渣和碱浸渣X射线衍射图谱
Fig.5 XRD patterns of As2S3 slag and basic leached slag
碱浸渣与硫化砷渣X射线衍射(XRD)结果如图5所示。由图5可知,硫化砷渣呈无定型态,碱浸渣中含有硫 化铜。
2.2 硫化砷渣浸出动力学
2.2.1 碱浸过程中As浸出率与时间的关系
在不同温度下,测得砷浸出率随反应时间的关系如图6所示。
从图6可知,砷浸出率随反应时间延长和反应温度增加而增加,两者呈线性关系;当反应时间为60 min,反应温度分别为25,40,55,70和90 ℃时,砷浸出率分别达到92.72%,94.50%,95.00%,95.50%和96.40%。
2.2.2 浸出动力学
硫化砷渣中固体As2S3与NaOH溶液反应,属于液固相反应,而且As2S3与NaOH反应无固相产物生成,
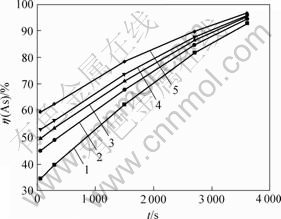
θ/℃: 1—25; 2—40; 3—55; 4—70; 5—90
图6 不同温度下As浸出率η(As)随反应时间的变化关系
Fig.6 Effect of reaction time on leaching rate of As at different temperatures
其反应及相变表示如下:
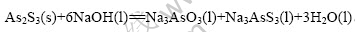
上述反应可认为反应是在固体颗粒As2S3表面进行的液-固相非催化反应。液-固相非催化反应最常见的反应模型为收缩未反应芯模型[11-14],简称为缩芯模型。缩芯模型又分为粒径不变缩芯模型和颗粒缩小缩芯模型。
粒径不变缩芯模型的特点是有固相产物层生成,反应过程中颗粒粒径不变。颗粒缩小缩芯模型的特点是:在反应过程中,反应物颗粒不断缩小,无固相产物层,产物溶于溶液中。
硫化砷与氢氧化钠反应生成极易溶于水的亚砷酸钠和硫代亚砷酸钠,可从颗粒缩小缩芯模型研究其反应动力学。当为流体滞流膜扩散控制时,颗粒缩小缩芯模型动力学方程为:
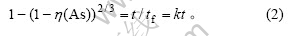
当反应为化学反应控制时,动力学方程为:
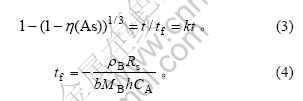
式中:η(As)为反应物浸出率;t为反应时间;tf为完全反应时间;ρB为固体反应物密度;Rs为固体颗粒初始反应半径;b为固体反应物计量系数;MB为固体反应物相对分子质量;h为反应速率常数;CA为液体反应物浓度。对于某一固定体系,且流体反应物浓度CA近似不变时,tf可识为常数,1/tf则可表示为k(其中k为表观反应速率常数)。
根据图6,作1-(1-η(As))2/3与反应时间的曲线,结果如图7所示;根据图7求出不同温度下各直线斜率即k,作ln k与1/T关系图,如图8所示。

θ/℃: 1—25; 2—40; 3—55; 4—70; 5—90
图7 不同温度下1-(1-η(As))2/3与反应时间的关系
Fig.7 Relationship between 1-(1-η(As))2/3 and reaction time at different temperatures

图8 ln k与1/T关系图
Fig.8 Relationship between ln k and 1/T
从图7可知,1-(1-η(As))2/3与反应时间呈良好线性关系,相关系数r均大于0.999 8,说明硫化砷渣碱浸过程中As2S3在NaOH溶液中的反应为收缩未反应芯扩散控制。反应为扩散控制时,一般反应温度对砷浸出率影响较小,这与反应温度对浸出率影响实验结果一致。
在化学反应中,反应速度常数k是温度的函数,温度对反应速度常数的影响可用阿累尼乌斯公式 表示:

式中:k为反应速度常数;k0为频率因子;E0为活化能;T为热力学温度;R为气体常数。根据图7求斜率得表观活化能E0为3.682 kJ/mol,求截距得频率因子k0为1.868×103,即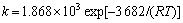 。
。
一般地,当反应活化能小于62.78 kJ/mol时,反应速度很快[15]。硫化砷渣中As2S3与NaOH反应活化能低,说明硫化砷渣中As2S3易溶于氢氧化钠。但是,由于反应物中CuS等不溶物的包裹作用和生成物的扩散阻碍,使其反应速度减慢。
3 结 论
a. 采用氢氧化钠浸出硫化砷渣,当反应温度为 90 ℃,固液比为1?6,反应时间为1.5 h,NaOH与As2S3的摩尔比为7.2?1时,砷浸出率达到95.90%,铜浸出率为0.087%。
b. 经过氢氧化钠浸,渣中Cu和Bi质量分数分别从10.90%和1.85%增加到50.00%和10.63%,铜和铋得到高度富集。
c. 硫化砷渣碱浸过程中As2S3在NaOH溶液中的反应为收缩未反应芯扩散控制,其动力学方程式为:
1-(1-η(As))2/3=kt。
其中: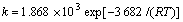 。
。
参考文献:
[1] 陈敬军, 蒋柏泉, 王 伟. 除砷技术现状与进展[J]. 江西化工, 2004(2): 1-4.
CHEN Jing-jun, JIANG Bo-quan, WANG Wei. Status and progress of arsenic removal[J]. Jiangxi Chemical Engineering, 2004(2): 1-4.
[2] Katsoyiannis I A, Zouboulis A I. Application of biological processes for the removal of arsenic from groundwater[J]. Water Research, 2004, 38(1): 17-26.
[3] Dutre V, Andecasteele C. Solidification/stabilization of hazardous arsenic containing waste from a copper refining process[J]. Journal of Hazardous Materials, 1995, 40(1): 55-68.
[4] 朱义年, 张 华, 梁延鹏, 等. 砷酸钙化合物的溶解度及其稳定性随pH值的变化[J]. 环境科学学报, 2005, 25(12): 1652-1660.
ZHU Yi-nian, ZHANG Hua, LIANG Yan-peng, et al. Dependence of solubility and stability of calcium arsenates on pH value[J]. Acta Scientiae Circumstantiae, 2005, 25(12): 1652-1660.
[5] 张荣良, 丘克强, 谢永金, 等. 铜冶炼闪速炉烟尘氧化浸出与中和脱砷[J]. 中南大学学报: 自然科学版, 2006, 37(1): 73-78.
ZHANG Rong-liang, QIU Ke-qiang, XIE Yong-jin, et al. Treatment process of dust from flash smelting furnace at copper smelter by oxidative leaching and neutralization process from leaching solution[J]. Journal of Central South University: Science and Technology, 2006, 37(1): 73-78.
[6] 刘昌勇. 贵溪冶炼厂亚砷酸生产工艺[J]. 有色冶炼, 1998(2): 8-10.
LIU Chang-yong. Arsenous acid production in Guixi Smelter[J]. Non-Ferrous Smelting, 1998(2): 8-10.
[7] 陈维平, 李仲英, 边可君, 等. 湿式提砷法在处理工业废水及废渣中的应用[J]. 中国环境科学, 1999, 19(4): 310-312.
CHEN Wei-ping, LI Zhong-ying, BIAN Ke-jun, et al. Application of wet-method for extracting arsenic in treating industrial wastewater and residues[J]. China Environmental Science, 1999, 19(4): 310-312.
[8] 田文增, 陈白珍, 仇勇海. 有色冶金工业含砷物料的处理及利用现状[J]. 湖南有色金属, 2004, 20(6): 11-15.
TIAN Wen-zeng, CHEN Bai-zhen, QIU Yong-hai. Review of As-containing materials treatment and utilization in nonferrous metallurgy industry[J]. Hunan Nonferrous Metals, 2004, 20(6): 11-15.
[9] 郑雅杰, 王 勇, 赵攀峰. 一种利用含砷废水制备亚砷酸铜和砷酸铜的方法: 中国, 200610032456.1[P]. 2006-10-25.
ZHENG Ya-jie, WANG Yong, ZHAO Pan-feng. A method of producing arsenite copper and arsenate copper from waste acid contained As: CN 200610032456.7[P]. 2006-10-25.
[10] 陈白珍, 龚竹青. 硫酸铜结晶母液制备砷酸铜的工艺技术[J]. 中南工业大学学报: 自然科学版, 2000, 31(4): 300-302.
CHEN Bai-zhen, GONG Zhu-qing. A technology of preparing arsenate copper from the mother liquid of copper sulfide[J]. Journal of Central South University of Technology: Natural Science, 2000, 31(4): 300-302.
[11] Tkacovo K, Balaz P. Selective leaching of zinc from mechanically activated complex Cu-Pb-Zn concentrate[J]. Hydrometallurgy, 1993, 33(3): 291-300.
[12] 朱炳辰. 化学反应工程[M]. 北京: 化学工业出版社, 1993.
ZHU Bing-chen. Chemical reaction engineering[M]. Beijing: Chemical Industry Press, 1993.
[13] Ekinci Z, Colak S, Cakici A. Technical note leaching kinetics of sphalerite with pyrite in chloride saturated water[J]. Minerals Engineering, 1998, 11(3): 279-283.
[14] Breed A W, Hansford G S. Studies on the mechanism and Kinetics of bioleaching[J]. Minerals Engineering, 1999, 12(4): 383-392.
[15] 傅献彩, 陈瑞华. 物理化学: 下册[M]. 北京: 人民教育出版社, 1979.
FU Xian-cai, CHEN Rui-hua. Physical chemistry: 2nd Volume[M]. Beijing: People Education Press, 1979.
收稿日期:2007-04-23;修回日期:2007-06-25
基金项目:广东省创新基金资助项目(200501045)
通信作者:郑雅杰(1959-),男,湖南常德人,教授,博士生导师,从事冶金、材料和环境保护研究;电话:0731-8836285;E-mail: zzyyjj01@yahoo.com.cn