CaCO3在铝酸钠溶液中的反苛化作用
李小斌,郑洁,齐天贵,王丹琴,张海宝
(中南大学 冶金与环境学院,湖南 长沙,410083)
摘要:在热力学计算的基础上,研究CaCO3在铝酸钠溶液体系中发生反苛化反应的规律。热力学计算表明,低温条件下,CaCO3与游离苛性碱形成Ca(OH)2的反苛化程度较小,而其与Al(OH)4-形成3CaO·Al2O3·6H2O(C3AH6)的反苛化反应显著。CaCO3在纯NaOH溶液中反苛化反应的反应率小于5%,而在铝酸钠溶液中其反苛化率可达50%以上,说明Al(OH)4-对CaCO3的反苛化具有显著的促进作用;CaCO3的反苛化率随温度的升高而升高,随铝酸钠溶液苛性比αk的升高呈先升高后降低的规律,当αk为3时,出现极大值;常压条件下,CaCO3在铝酸钠溶液中发生反苛化反应形成C3AH6,而造成氧化铝损失。
关键词:碳酸钙;反苛化;铝酸根离子(Al(OH)4-);六水合铝酸三钙(C3AH6)
中图分类号:TF821 文献标志码:A 文章编号:1672-7207(2013)07-2663-06
Anti-causticization of calcium carbonate in sodium aluminate solution
LI Xiaobin, ZHENG Jie, QI Tiangui, WANG Danqin, ZHANG Haibao
(School of Metallurgical and Environment, Central South University, Changsha 410083, China)
Abstract: Anti-causticization of calcium carbonate in sodium aluminate solution was studied. The thermodynamic calculation result shows that the degree of anti-causticization reaction of CaCO3 with NaOH solution to form Ca(OH)2 is low at low temperatures, while that of CaCO3 with Al(OH)4- to form 3CaO·Al2O3·6H2O (C3AH6) is significant. The reaction rate of CaCO3 is less than 5% in pure sodium hydroxide solution and more than 50% in sodium aluminate solution, indicating that Al(OH)4- anion can significantly promote the anti-causticization of calcium carbonate in sodium aluminate solution. The reaction rate of anti-causticization improves with the increase of temperature, increasing first and then decreasing with the increase of the mole ratio of Na2O to Al2O3 (αk), and reaches the maximum with αk of approximate 3. The results also show that C3AH6 is formed in the anti-causticization of CaCO3 in sodium aluminate solution under the atmospheric condition, causing the loss of alumina.
Key words: calcium carbonate; anti-causticization; Al(OH)4-; tricalcium aluminate hexahydrate(C3AH6)
从广义上讲,拜耳法生产氧化铝过程中的反苛化是指将铝酸钠溶液中的苛性碱转化为碳酸钠的过程,它不仅包括矿石及石灰添加剂中各种碳酸盐与铝酸钠溶液形成碳酸钠的过程,也包括腐植酸钠等有机物的分解[1]和空气搅拌过程中带入CO2造成苛性碱转化为碳酸钠的过程,但在实际生产过程中各种原料中的碳酸盐是引起反苛化作用的主要原因[2]。碳酸盐的反苛化对氧化铝生产的危害是多方面的:它使溶液部分苛性碱转化为碳酸钠,不利于矿石中氧化铝的溶出;溶液中碳酸钠浓度的升高,会引起溶液黏度升高,导致赤泥沉降分离困难[3-4],并使种分过程分解率降低[5];碳酸钠在母液蒸发时析出还会降低蒸发换热器的传热效率,增加蒸发能耗[6]。CaCO3(石灰石)与铝酸钠溶液组分反应形成碳酸钠的反应是典型的反苛化反应。普遍认为[7-10],拜耳法生产氧化铝系统中,反苛化反应主要是游离NaOH与CaCO3作用的结果,该反应仅在高温(>150 ℃)[10]条件下方可显著进行;而在低温(<100 ℃)条件下,CaCO3的反苛化反应则十分微弱,一直被忽略。因此,在相当一部分氧化铝生产企业中,以CaCO3为主要组成的苛化渣直接混入赤泥洗涤系统中经沉降分离后随赤泥外排。但是,即使在低温铝酸钠溶液中,CaCO3反苛化也十分明显,且伴随着氧化铝的损失,其原因有待深入研究。因此,本文作者在热力学分析的基础上,对CaCO3的反苛化作用进行实验研究,以期进一步明确铝酸钠溶液中CaCO3反苛化规律,为控制生产流程中的反苛化作用提供理论基础。
1 热力学分析
碳酸钙在高温条件下的反苛化反应已有广泛的研究,为了进一步明确其在低温(常压)条件下的反苛化反应行为,有必要对碳酸钙低温铝酸钠溶液中的反苛化反应热力学进行深入分析。在Na2O-CaO-Al2O3- CO2-H2O体系中,稳定存在的含钙化合物主要有Ca(OH)2,CaCO3和3CaO·Al2O3·6H2O,CaCO3与该体系中的游离苛性碱及铝酸根反应形成Na2CO3的反苛化反应,可由反应(1)~(3)表示。
CaCO3(s)+2OH-(aq)  CO32-(aq)+Ca(OH)2 (s) (1)
CO32-(aq)+Ca(OH)2 (s) (1)
3Ca(OH)2(s)+2Al(OH)4-(aq)  3CaO·Al2O3·6H2O(s)+2OH-(aq) (2)
3CaO·Al2O3·6H2O(s)+2OH-(aq) (2)
3CaCO3(s)+4OH-(aq)+2Al(OH)4-(aq)  3CaO·Al2O3·6H2O(s)+3CO32-(aq) (3)
3CaO·Al2O3·6H2O(s)+3CO32-(aq) (3)
采用经典热力学计算方法,计算得到不同温度下的标准吉布斯自由能变化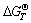 及反应平衡常数K1~K3,如表1所示。其中,热力学计算使用到的化合物及离子的热力学数据来自文献[11-13]。
及反应平衡常数K1~K3,如表1所示。其中,热力学计算使用到的化合物及离子的热力学数据来自文献[11-13]。
一般认为,在高压溶出过程中,反应(1)所示的碳酸钙与铝酸钠溶液中游离苛性碱之间的反苛化反应是溶液中碳酸钠浓度升高的主要原因。在常压条件下,若固体反应物的活度取1,溶液中NaOH,NaAl(OH)4,Na2CO3等的活度系数(rx,x为NaOH,Na2CO3等)采用彭小奇等[14]提出的NaOH-NaAl(OH)4-Na2CO3-H2O体系中活度系数的计算方法计算,由反应平衡常数表达式(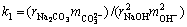 )及表1中的数据可计算出不同温度下反应(1)达到平衡时Na2CO3的平衡浓度。由此得到的CaCO3与铝酸钠溶液中游离苛碱发生反苛化反应的平衡状态图如图1所示。
)及表1中的数据可计算出不同温度下反应(1)达到平衡时Na2CO3的平衡浓度。由此得到的CaCO3与铝酸钠溶液中游离苛碱发生反苛化反应的平衡状态图如图1所示。
由图1可以看出,在低温(373 K)条件下,铝酸钠溶液的苛碱浓度(Na2Ok)及苛性分子比(αk)对CaCO3与游离苛碱之间的反苛化反应影响明显,苛碱浓度升高或苛性分子比增大,CaCO3在平衡状态图中的稳定区减小,碳酸钠平衡浓度升高。在323~373 K内,升高温度CaCO3与游离苛碱之间的反苛化反应加强,但温度对该反应平衡的影响不显著。由图1可知,即使在温度为373 K、苛碱浓度200 g/L、苛性分子比为3的条件下,CaCO3与铝酸钠中游离苛碱之间的反苛化反应达到平衡时,碳酸钠的平衡浓度仍小于3 g/L。说明常压条件下,CaCO3与铝酸钠溶液中苛性碱之间并不发生显著的反苛化反应。
表1 反应(1)~(3)的标准吉布斯自由能变化及反应平衡常数
Table 1 Standard Gibbs free energy and reaction equilibrium constant of reactions (1)-(3)
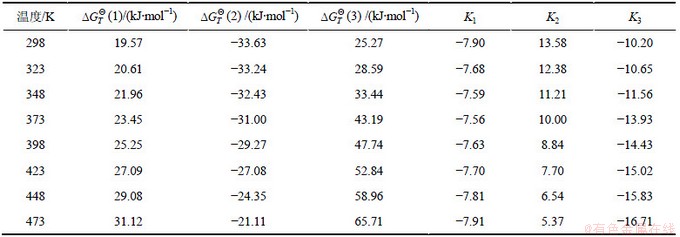
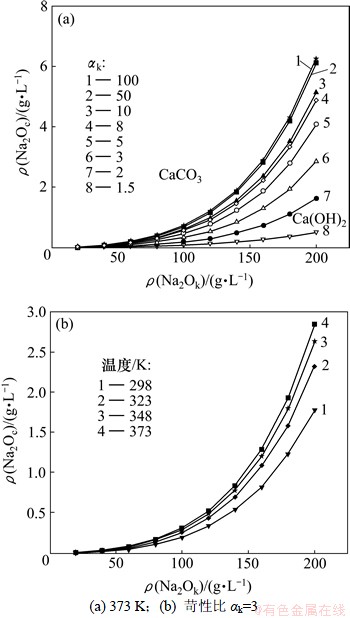
图1 CaCO3与铝酸钠溶液中游离NaOH之间反苛化反应平衡状态图
Fig.1 Equilibrium diagram of anti-causticization of CaCO3 with free NaOH in aluminate solution
采用相同的计算方法,对反应(3)所示的CaCO3与铝酸钠反应形成C3AH6和Na2CO3的反苛化反应进行热力学平衡计算,得到不同条件下Na2CO3的平衡浓度及CaCO3与C3AH6的平衡状态图,如图2所示。
由图2可知,在常压条件下,CaCO3与铝酸钠反应形成C3AH6和Na2CO3的反苛化反应达到平衡时,其碳酸钠的平衡浓度随苛碱浓度的升高和反应温度的降低而升高;在相同温度和苛碱浓度条件下,碳酸钠平衡浓度随苛性分子比的增加呈现先增加后减小的规律,其平衡浓度在苛性分子比为3左右时达到最大。将图2与图1对比可知,在相同温度和铝酸钠溶液组成条件下,CaCO3发生反应(3)所示的反苛化反应平衡时的碳酸钠平衡浓度远高于反应(1)达到平衡时碳酸钠的平衡浓度。这说明在低温条件下,CaCO3与Al(OH)4-和OH-反应形成C3AH6和Na2CO3的反苛化反应会显著发生,它不仅使铝酸钠溶液中碳酸钠浓度升高,也造成生产系统氧化铝的损失。
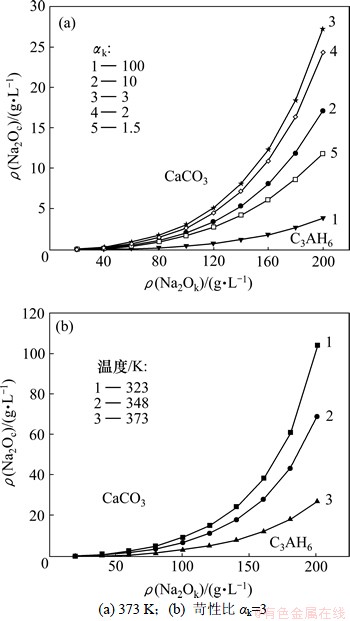
图2 CaCO3与铝酸根之间反苛化反应的平衡状态图
Fig.2 Equilibrium diagram of anti-causticization of CaCO3 with Al(OH)4-
在温度和溶液苛性分子一定的条件下,图1和图2中碳酸钠的平衡浓度曲线将图中区域分别划分为CaCO3与Ca(OH)2的稳定区和CaCO3与C3AH6的稳 定区。当温度为373 K、铝酸钠溶液苛性分子比为3时,将图1和2中CaCO3的稳定区进行叠加如图3所示。
由图3可以看出,CaCO3与游离苛碱发生反苛化反应生成Ca(OH)2时,A+B为CaCO3的稳定区,C为Ca(OH)2的稳定区;当溶液中有Al(OH)4-参与反应时,反苛化反应平衡固相为CaCO3和C3AH6,此时CaCO3的稳定区减小为A,C3AH6的稳定区为B+C。即在该条件下,由于体系中Al(OH)4-参与反苛化反应,CaCO3的稳定区减小,其反苛化程度显著增加。
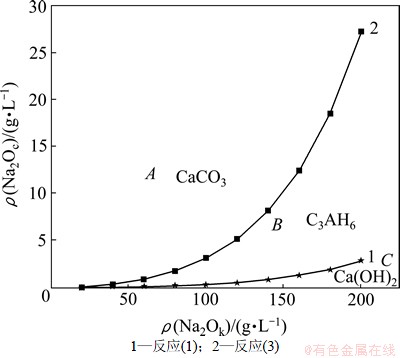
图3 不同反苛化反应中CaCO3稳定区比较
Fig.3 Comparision of stability region of CaCO3 in different anti-causticization
2 实验原料和方法
称取一定量的分析纯CaCO3至150 mL钢弹中,加入100 mL氢氧化钠溶液(分析纯)或铝酸钠溶液(分析纯NaOH和工业Al(OH)3配制而成),再加入2个直径为15 mm和2个直径为8 mm的钢球加强搅拌,将加入反应物料并密封后的钢弹放入已预热至一定温度的DY-8型低压釜(甘油为加热介质)或XYF-6钢弹型高压溶出装置(熔盐为加热介质)中,反应一定时间后,取出、过滤、洗涤,用酸碱滴定法分析反应后铝酸钠溶液中氧化铝和苛性碱浓度,用双指示剂法分析反应后NaOH溶液中碳酸钠浓度,固相烘干后进行X线衍射分析(Rigaku-TTRⅢ型X线衍射仪)。
3 实验结果与讨论
3.1 CaCO3与NaOH溶液的作用
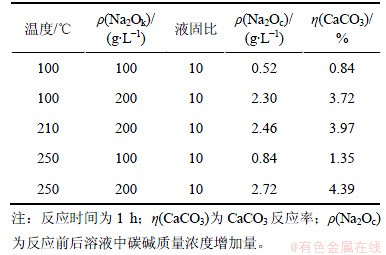
在高温高压溶出过程中,CaCO3很容易与溶液中的游离Na2Ok发生反苛化作用[2]。但热力学计算表明,在常压条件下该反苛化反应不明显,为明确碳酸钙与游离苛碱的反苛化规律,对不同温度下CaCO3与纯NaOH溶液之间的反应进行研究,其结果如表2所示。
从表2可以看出,在相同液固比条件下,CaCO3与纯NaOH溶液发生反苛化反应形成碳酸钠的过程中,提高温度和增加NaOH浓度均使碳酸钙反苛化率升高。但从碳酸钙的反应率看,即使在250 ℃的高温下,碱质量浓度高达200 g/L时,碳酸钙的反苛化反应率仅4.39%,这说明CaCO3在纯NaOH溶液中的反苛化反应不明显。这与图1的热力学计算结果相吻合。
表2 CaCO3在纯NaOH溶液中反苛化反应的结果
Table 2 Result of anti-causticization of CaCO3 in sodium hydroxide solution
3.2 CaCO3在铝酸钠溶液中的反苛化作用
热力学计算表明,在铝酸钠溶液中,由于Al(OH)4-参与CaCO3的反苛化反应形成C3AH6,使溶液中碳酸钠平衡浓度显著升高,为明确Al(OH)4-在反苛化反应中的作用,实验研究了CaCO3在铝酸钠溶液中的反苛化反应规律,结果如表3和表4所示。
由表3可以看出,在100 ℃条件下,CaCO3加入到铝酸钠溶液中反应一定时间后,溶液中苛性碱浓度和氧化铝浓度显著降低,而碳酸钠浓度升高,说明CaCO3与铝酸钠溶液在上述条件下可发生显著的反苛化反应。对比表3和表2可知,在相同的反应温度和液固比条件下,碳酸钙在铝酸钠溶液中的反苛化率远高于其在纯NaOH溶液中的反苛化率,这说明在较低温度下,CaCO3的反苛化主要是由它与Al(OH)4-及OH-之间反应形成3CaO·Al2O3·6H2O和Na2CO3引起的。由表4可以看出,在250 ℃的高温反应条件下,CaCO3在铝酸钠溶液中的反苛化率显著高于低温下的反苛化率,而从热力学计算结果看,反应(1)表示的反苛化反应随温度的升高而加强,而反应(3)表示的反苛化反应随温度的变化趋势与此相反,反应(1)可能是反苛化的主要反应,这与在高温条件下3CaO·Al2O3·6H2O不稳定[15-16]相吻合。对比表4和表2可知,在碳酸钙配量相同时,CaCO3在游离碱浓度相近的铝酸钠溶液中的反苛化率是其在纯NaOH中反苛化率的10倍左右,这说明在高温条件下Al(OH)4-对CaCO3的反苛化反应仍具有显著的促进作用,且这种促进作用并不是由于形成C3AH6引起的。
表3 100 ℃下CaCO3在铝酸钠溶液中反苛化反应结果
Table 3 Result of anti-causticization of CaCO3 in sodium aluminate solution at 100 ℃
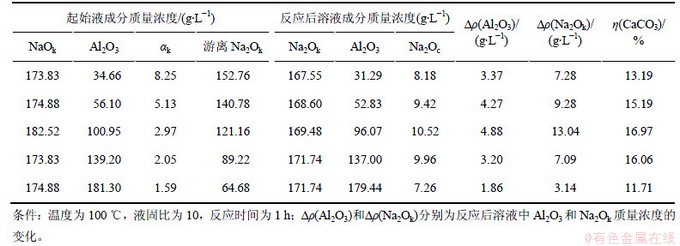
表4 250 ℃下CaCO3在铝酸钠溶液中反苛化作用的结果
Table 4 Result of anti-causticization of CaCO3 in sodium aluminate solution at 250 ℃
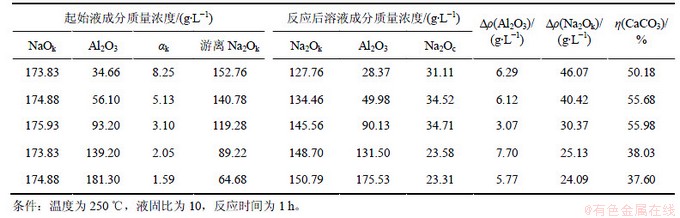
在苛碱浓度相同的条件下,CaCO3在铝酸钠溶液中的反苛化率及溶液中的碳酸钠质量浓度随着αk的减小呈先增加后减小的规律,在αk为3左右时,其反苛化作用最显著,这与图2的热力学计算结果相吻合。这一规律与反应(3)达到平衡时,碳酸钠平衡浓度与溶液中Al(OH)4-及OH-的定量关系有关,根据反应(3)热力学平衡常数K3与反应物浓度之间的表达式,在一定温度下,反应平衡时碳酸钠平衡浓度与Al(OH)4-浓度的平方及OH-浓度的4次方成正比,苛碱浓度一定时,其平衡碳酸钠浓度在αk=3时出现极大值,也就是说在苛碱浓度一定时,碳酸钙在αk为3左右的铝酸钠溶液中的反苛化反应最显著。
3.3 CaCO3反应物相分析
为了进一步明确CaCO3与铝酸钠溶液中发生反苛化反应过程中的物相变化,对CaCO3在温度80 ℃、液固比为10的条件下,碳酸钙在铝酸钠溶液中反应1 h所得固相产物进行物相分析,结果如图4所示。
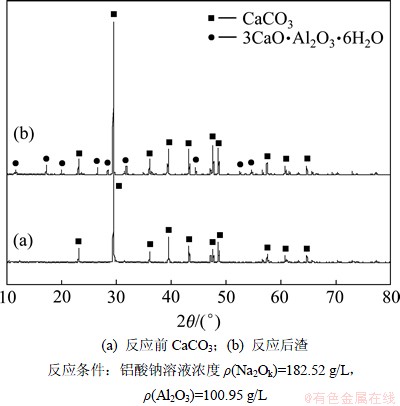
图4 CaCO3及其在铝酸钠溶液中反应后固体产物的XRD衍射图谱
Fig.4 XRD patterns of CaCO3 and reaction products of CaCO3 in sodium aluminate solution
从图4可以看出,CaCO3与铝酸钠溶液反应后渣中除有CaCO3的特征衍射峰外,还出现了3CaO·Al2O3·6H2O的特征峰,说明低温条件下CaCO3与铝酸钠溶液反苛化反应的固相产物为3CaO·Al2O3·6H2O,这与由表3中数据计算得到的参加反应的CaCO3与溶液中损失氧化铝的摩尔比约为3相一致。
4 结论
(1) CaCO3在铝酸钠溶液中可发生显著的反苛化反应,其反苛化率远高于它在NaOH溶液中的反苛化率,Al(OH)4-对反苛化有显著的促进作用。
(2) CaCO3的反苛化率随铝酸钠溶液苛性比αk的减小呈现先升高后降低的规律,在分子比为3左右时出现极大值。
(3) CaCO3在铝酸钠溶液中的反苛化随温度的升高而加强;常压条件下,CaCO3在铝酸钠溶液中发生反苛化反应形成3CaO·Al2O3·6H2O,从而造成氧化铝的损失。
参考文献:
[1] Power G, Loh J. Organic compounds in the processing of lateritic bauxites to alumina, Part 1: Origins and chemistry of organics in the Bayer process[J]. Hydrometallurgy, 2010, 105(1/2): 1-29.
[2] 杨重愚. 氧化铝生产工艺学[M]. 北京: 冶金工业出版社, 1982: 64-65.
YANG Zhongyu. Technology of alumina production[M]. Beijing: Metallurgical Industry Press, 1982: 64-65.
[3] Jones F, Farrow J B, van Bronswijk W. Effect of caustic and carbonate on the flocculation of haematite in synthetic Bayer liquors[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1998, 142(1): 65-73.
[4] 刘桂华, 刘云峰, 李小斌, 等. 拜耳法溶出过程降低赤泥碱耗[J]. 中国有色金属学报, 2006, 16(3): 555-559.
LIU Guihua, LIU Yunfeng, LI Xiaobin, et al. Reducing loss of soda in red mud in process of Bayer digestion[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(3): 555-559.
[5] 彭志宏, 邓永春, 周秋生, 等. 无机盐杂质对铝酸钠溶液晶种分解率的影响[J]. 矿业工程, 2010, 30(1): 57-61.
PENG Zhihong, DENG Yongchun, ZHOU Qiusheng, et al. Influence of inorganic salt impurities on seed decomposition rate in sodium aluminate solutions[J]. Mining And Metallurgical Engineering, 2010, 30(1): 57-61.
[6] 彭志宏, 陈科云, 李小斌, 等. 铝酸钠溶液蒸发过程中的结垢与防垢[J]. 中南大学学报: 自然科学版, 2008, 39(1): 69-74.
PENG Zhihong, CHEN Keyun, LI Xiaobin, et al. Scaling and anti-scale in evaporation process of sodium aluminate solution[J]. Journal of Central South University: Science and Technology, 2008, 39(1): 69-74.
[7] Rosenberg Steven P, Tichbon Wayne, Aboagye Alex R, et al. Process for the removal of anionic impurities from caustic aluminate solution: Australia, US7691348 B2[P]. 2010-04-06.
[8] Philip R S, James W D, Catherine H. Bayer Causticisation: Australia, US 2003/0129125 A[P]. 2003-07-10.
[9] Whittington B I. The chemistry of CaO and Ca(OH)2 relating to the Bayer process[J].Hydrometallurgy, 1996, 43(1/2/3): 13-35.
[10] Max W, Franklin V. Impact of Bayer process liquor impurities on causitization[J]. Industrial and Engineering Chemistry Research, 2007, 46(15): 5094-5099.
[11] Bunah A G. 矿物学中的热力学方法[M]. 夏林圻, 译. 北京: 地质出版社, 1982: 136-142.
Bunah A G. Thermodynamic method for mineral[M]. XIA Linyin, trans. Beijing: Geology Press, 1982: 136-142.
[12] 巴布什金V I, 马特维耶夫G M. 硅酸盐热力学[M]. 蒲新诚, 曹建华, 译. 北京: 中国建筑工业出版社, 1983: 254-262.
Babushkin V I, Matveev G M. Thermodynamics of silicates[M]. PU Xincheng, CAO Jianhua, trans. Beijing: China Architecture & Building Press, 1983: 254-262.
[13] 杨显万, 何蔼平. 高温水溶液热力学数据计算手册[M]. 北京: 科学出版社, 1983: 20-105.
YANG Xianwan, HE Aiping. Thermodynamic calculations manual of high temperature solution[M]. Beijing: Science Press, 1983: 20-105.
[14] 彭小奇, 宋国辉, 宋彦坡, 等. NaOH-NaAl(OH)4-Na2CO3-H2O体系活度因子的计算模型[J]. 中国有色金属学报, 2009,19(7): 1332-1337.
PENG Xiaoqi, SONG Guohui, SONG Yanpo, et al. Calculation model of activity coefficient for NaOH-NaAl(OH)4-Na2CO3-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(7): 1332-1337.
[15] Whittington B I, Cardile C M. The chemistry of tricalcium aluminate hexahydrate relating to the Bayer industry[J]. International Journal of Mineral Processing, 1996, 48(1/2): 21-38.
[16] WANG Shaona, ZHENG Shili, ZHANG Yi. Stability of 3CaO·Al2O3·6H2O in KOH+K2CO3+H2O system for chromate production[J]. Hydrometallurgy, 2008, 90(2/3/4): 201-206.
(编辑 赵俊)
收稿日期:2012-06-09;修回日期:2012-09-10
基金项目:中央高校基本科研业务费专项资金资助(2010QZZD004)
通信作者:齐天贵(1982-),男,河南南阳人,博士,从事有色金属冶金研究;电话:0731-88830453;E-mail: qitiangui@csu.edu.cn