
Synthesis of ZrO2-SiC composite powder and effect of its addition on properties of Al2O3-C refractories
MA Bei-yue(马北越), YU Jing-kun(于景坤)
School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 23 January 2007; accepted 18 May 2007
Abstract: ZrO2-SiC composite powder was synthesized by carbothermal reduction of zircon in argon atmosphere, and it was used as the additive to prepare Al2O3-C refractories. The effects of heating temperature on the synthesis process and the addition of the synthesized composite powder on the properties of the Al2O3-C refractories were investigated. The results show that the synthesized composite powder can be easily obtained by heating the mixture of zircon and carbon black at 1 873 K for 4 h in argon atmosphere, and the relative contents of ZrO2 and SiC in sample reach about 83.7% and 16.3%, respectively. The bulk density, crushing strength and thermal shock resistance of the Al2O3-C refractories can be improved obviously by the addition of the synthesized ZrO2-SiC composite powder.
Key words: ZrO2-SiC composite powder; carbothermal reduction; crushing strength; thermal shock resistance; Al2O3-C refractories
1 Introduction
Al2O3-C refractories have been widely used in high temperature metallurgical processes including iron making, steel making and continuous casting owing to the refractories’ excellent properties[1]. In order to satisfy the development of continuous casting and hot iron pretreatment technologies, the properties of the refractories need to improve. The oxidation and corrosion resistance of the Al2O3-C refractories can be improved obviously by the addition of CaB6[2], B4C[3] and the synthesized Al2O3-SiC composite from clay[4]. In addition, ZrO2 and SiC powders can also improve the properties of the Al2O3-C refractories due to their high toughness, wear and corrosion resistance[5-6]. However, high price of ZrO2 and SiC especially ZrO2 micro powders increases the cost of products and influences the application of them in the refractories.
In terms of economy and efficiency, the carbothermal reduction method is the best choice to synthesize composite powder[7-8]. In recent years, some works have been focused on the use of the carbothermal reduction method to synthesize composites such as Al2O3-SiC[9], mullite-SiC[10], mullite-ZrO2-SiC[10], Al2O3-mullite-SiC[11], Al2O3-ZrO2-SiC[7] and Al2O3- Sialon-SiC[12]. The starting materials of synthesizing the composites are mostly natural minerals such as pyrophyllite[13], clay[9, 11] and andalusite[14], which can prepare the excellent composites and make the natural resources be utilized fully. So far, there is no report on the synthesis of the ZrO2-SiC composite powder from zircon.
In this study, ZrO2-SiC composite powder is synthesized by carbothermal reduction of zircon in argon atmosphere. The effects of the heating temperature on the phase composition, relative content and microstructure of the synthesized ZrO2-SiC composite powder as well as its addition on the bulk density, apparent porosity, crushing strength and thermal shock resistance of the Al2O3-C refractories are investigated.
2 Experimental
Zircon (<44 μm) and carbon black (<30 μm) were used as the starting materials, and the chemical composition of zircon is listed in Table 1. In addition, the mass fraction of C in carbon black and the volume frac-tion of argon gas are 98.0% and 99.99%, respectively.
Table 1 Chemical composition of zircon (mass fraction, %)

According to the reaction (1), the mass ratio of zircon to carbon black is 100/20:
ZrSiO4(s)+3C(s)=ZrO2(s)+SiC(s)+2CO(g) (1)
Zircon and carbon black were weighed in terms of this mass ratio, mixed in a ball mill for 24 h, pressed at 100 MPa into the samples with size of d 20 mm×5 mm, dried at 393 K for 24 h and then heated at 1 723, 1 773, 1 823 and 1 873 K for 4 h at a heating rate of 283 K/min, respectively. The flux of the argon gas remained at 1.5 L/min during the heat-up and reaction periods. After the desired reaction temperature reached, the system was cooled to1 273 K at a rate of 279 K/min and then cooled to room temperature in air. The samples heated at 1 723 -1 873 K were calcined at 973 K for 2 h to remove residual carbon. The phase composition and relative content of samples were determined by XRD(X-ray diffraction), and the microstructure was observed by SEM(scanning electronic microscope). The relative contents of phases in sample were estimated by
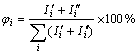 (2)
(2)
where 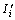 and
and  are the absolute intensity of diffraction peak of i phase from two reflecting surface, φi is the relative content of i phase in sample.
are the absolute intensity of diffraction peak of i phase from two reflecting surface, φi is the relative content of i phase in sample.
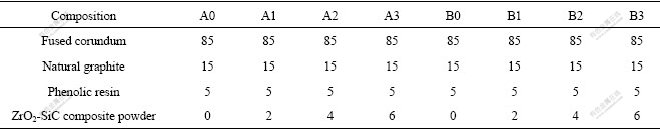
The synthesized ZrO2-SiC composite powder was used as the additive to prepare the Al2O3-C refractories. The starting materials were weighed in terms of the compositions as listed in Table 2, mixed fully, pressed at 200 MPa into the samples with size of d 50 mm×20 mm, dried at 523 K for 24 h and then sintered at 1 673 K for 2 h covered up with carbon. The sintered samples at 1673 K were put into the high temperature furnace (1 473 K) to investigate the thermal shock resistance. In this study, the thermal shock resistance of the refractories was udged by comparing the conservation rate of strength. Moreover, the conservation rate of strength is equal to the crushing strength before thermal shock, and dividing the residual strength after thermal shock. In addition, the apparent porosity and bulk density of samples were measured.
Table 2 Composition of Al2O3-C refractories (mass fraction, %)
3 Results and discussion
3.1. Effect of heating temperature on synthesis of ZrO2-SiC composite powder
As shown in Fig.1 the synthesized ZrO2-SiC composite powder includes ZrO2, SiC and ZrSiO4 phases when the samples are heated at 1 723-1 823 K, and the diffraction peak intensity of SiC phase increases gradually with increasing the heating temperature. However, that of the ZrSiO4 phase weakens, and vanishes when the heating temperature rises to 1 873 K. During the heating process, the diffraction peak intensity of ZrO2 increases. In Fig.2 the relative contents of ZrO2 and SiC increase with increasing the heating temperature, while the content of ZrSiO4 decreases and becomes zero when the sample is heated at 1 873 K for 4 h, and the relative contents of ZrO2 and SiC in sample are about 83.7% and 16.3%, respectively.
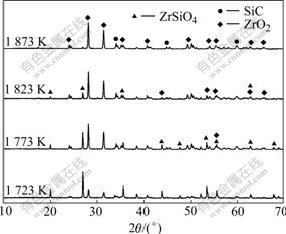
Fig.1 Phase compositions of samples heated at 1 723-1 873 K for 4 h
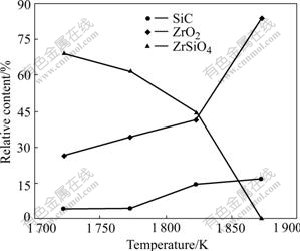
Fig.2 Effect of heating temperature on relative content of phases
Fig.3 shows the SEM image and EDS spectrum of the sample heated at 1 873 K for 4 h. They show that the formed particles are about 1 μm when zircon and carbon black are heated at 1 873 K for 4 h and the materials are composed of ZrO2 and SiC, perhaps because the initial formed SiC particles develop and grow up on the surface of ZrO2 matrix.
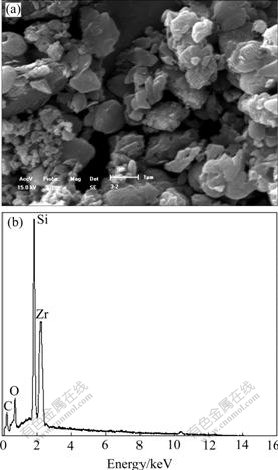
Fig.3 SEM image (a) and EDS spectrum (b) of sample heated at 1 873 K for 4 h
3.2 Thermodynamic analysis of carbothermal reduc- tion reaction process
ZrSiO4 can be decomposed and form ZrO2 and SiO2 during the process of heating the mixture of zircon and carbon black, and the chemical reaction equation can be written as
ZrSiO4(s)=ZrO2(s)+SiO2(s) (3)
The formed ZrO2, SiO2 and C cannot coexist at high temperature and can form SiC and ZrC during the heating process[15-16]:
SiO2(s)+C(s)=SiO(g)+CO(g) (4)
SiO(g)+2C(s)=SiC(s)+CO(g) (5)
SiO2(s)+3C(s)=SiC(s)+2CO(g) (6)
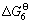 /(J?mol-1)=603 150-331.98T
/(J?mol-1)=603 150-331.98T
lg[p(CO)/pΘ]=8.67-15 750.39/T
ZrSiO4(s)+3C(s)=ZrO2(s)+SiC(s)+2CO(g) (7)
ZrO2(s)+C(s)=ZrO(g)+CO(g) (8)
ZrO(g)+2C(s)=ZrC(s)+CO(g) (9)
ZrO2(s)+3C(s)=ZrC(s)+2CO(g) (10)
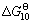 /(J?mol-1)=666 550-346.04T
/(J?mol-1)=666 550-346.04T
lg[p(CO)/pΘ]=9.04-17 406.00/T
ZrSiO4(s)+6C(s)=ZrC(s)+SiC(s)+4CO(g) (11)
Fig.4 shows the domain areas of the condensed phases in Zr-Si-C-O system plotted by the thermodynamic data for reactions (6) and (10). During the whole heating process, C in sample will react with SiO2 to form SiC (reactions (3)-(7)). When the partial pressure of CO(p(CO)) in the reacting furnace remains constant, the stability domain of the condensed phases changes from ZrO2(s)+SiC(s)+C(s) to ZrC(s)+SiC(s)+ C(s) with increasing the heating temperature. For example, when p(CO) is 1.0×105 Pa, the temperatures to form SiC and ZrC are about 1 816 K and 1 926 K, respectively. When the heating temperature remains constant, the domain area will change from ZrO2(s)+SiO2(s)+C(s) to ZrO2(s)+SiC(s)+C(s) and ZrC(s)+SiC(s)+C(s) with decreasing the p(CO).
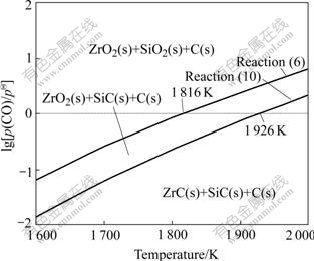
Fig.4 Domain areas of condensed phases in Zr-Si-C-O system
3.3. Effect of ZrO2-SiC composite powder addition on properties of Al2O3-C refractories
In Fig.5 the bulk density of samples tends to increase with increasing the synthesized composite powder. The bulk density of the sample by the addition of 4% ZrO2-SiC composite powder reaches the maximum, however, the apparent porosity decreases to the minimum, and their values are 2.79 g/cm3 and 18.8%, respectively.
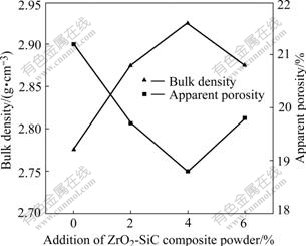
Fig.5 Effect of addition of ZrO2-SiC composite powder on bulk density and apparent porosity of samples sintered at 1 673 K for 2 h
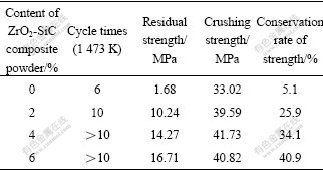
In Table 3 the cycle times of thermal shock, residual strength and conservation rate of strength of the samples increase with increasing the content of the ZrO2-SiC composite powder, while the crushing strength increases and then decreases, which indicates the strength of the Al2O3-C refractories can be improved by the addition of the ZrO2-SiC composite powder due to the high toughness of ZrO2 and SiC as well as their active effect on the sintering of refractories. However, the micro- flaws will become bigger due to the increasing of additive and the difference of thermal expansion coefficient between additive and refractories, which makes the crushing strength decrease. The conservation rate of strength of the sample with 6% ZrO2-SiC composite powder addition reaches the maximum, which shows the synthesized composite powder can also improve the thermal shock resistance of the Al2O3-C refractories.
Table 3 Effect of addition of ZrO2-SiC composite powder on properties of Al2O3-C refractories
The volume change of 3.5% (volume fraction) is accompanied by the reversible phase transformation of ZrO2 (Eqn.(12)) during the cooling process, and the amounts of micro-flaws come up around grain boundary. The shorter flaws extend mainly by dynamic expansion and the longer ones extend by quasi-static expansion. The edges and tips of the micro-flaws can be inactivated owing to the amounts of micro-flaws and their expansion forms, which can prevent the flaws from extending quickly and breaking of the materials, and further improve the thermal shock resistance of the Al2O3-C refractories.
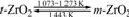 (12)
(12)
4 Conclusions
1) The ZrO2-SiC composite powder with the particle size of about 1 μm is synthesized by carbothermal reduction method in argon atmosphere. When heating the mixture of zircon and carbon black at 1 873 K for 4 h, the relative contents of ZrO2 and SiC in the synthesized composite powder are about 83.7% and 16.3%, respectively.
2) The bulk density, crushing strength and thermal shock resistance of the Al2O3-C refractories can be improved obviously by the addition of the synthesized ZrO2-SiC composite powder.
References
[1] CHAN C F, ARGENT B B, LEE W E. Influence of additives on slag resistance of Al2O3-SiO2-SiC-C refractory bond phases under reducing atmosphere [J]. J Am Ceram Soc, 1998, 81(12): 3177-3188.
[2] ZHENG S Q, MIN G H, ZOU Z D, TATSUYAMA C. High temperature oxidation of calcium hexaboride powders [J]. Mater Lett, 2004, 58(21): 2586-2589.
[3] LI Y Q, QIU T. Oxidation behavior of boron carbide powder [J]. Mater Sci Eng A, 2007, 444(1/2): 184-197.
[4] YU J K, LI H X, HIRAGUSHI K. Improvement of the corrosion resistance of refractories by adding Al2O3-SiC composites synthesized from natural minerals [J]. Taikabutsu, 1997, 49(11): 607.
[5] JIANG Y Z, GAO J F, LIU M F, WANG Y Y, MENG G Y. Fabrication and characterization of Y2O3 stabilized ZrO2 films deposited with aerosol-assisted MOCVD [J]. Solid State Ionics, 2007, 177(39/40): 3405-3410.
[6] YANG Y, YANG K, LIN Z M, LI J T. Mechanical-activation-assisted combustion synthesis of SiC [J]. Mater Lett, 2007, 61(3): 671-676.
[7] MARIAPPAN L, KANNAN T S, UMARJI A M. In situ synthesis of Al2O3-ZrO2-SiCw ceramic matrix composites by carbothermal reduction of natural silicates [J]. Mater Chem Phys, 2002, 75(1/3): 284-290.
[8] LIU X K, LUO F, ZHU D M, ZHOU W C. Microwave permittivity of SiC-Al2O3 composite powder prepared by sol-gel and carbothermal reduction [J]. Trans Nonferrous Met Soc China, 2006, 16(suppl.1): 494-497.
[9] HAN B Q, LI N. Preparation of β-SiC/Al2O3 composite from kaolinite gangue by carbothermal reduction [J]. Ceram Int, 2005, 31(2): 227-231.
[10] LIN Y J, TSANG C P. Fabrication of mullite/SiC and mullite/zirconia/SiC composites by “dual” in site reaction syntheses [J]. Mater Sci Eng A, 2003, 344(1/2): 168-174.
[11] ELIAS F N, KIMINAMI R A. Al2O3/mullite/SiC powders synthesized by microwave-assisted carbothermal reduction of kaolin [J]. Ceram Int, 2001, 27(7): 815-819.
[12] ZHANG H J, HAN B, LIU Z J. Preparation and oxidation of bauxite-based β-sialon-bonded SiC composite [J]. Mater Res Bull, 2006, 41(9): 1681-1689.
[13] YU J K, HIRAGUSHI K. Synthesis of Al2O3-SiC composite from pyrophyllite and clay and its application in carbon-containing refractories [J]. Taikabutsu, 1998, 50(7): 375-383.
[14] AMROUNE A, FANTOZZI G, DUBOIS J, DELOUME J P, DURAND B, HALIMI R. Formation of Al2O3-SiC powder from andalusite and carbon [J]. Mater Sci Eng A, 2000, 290(1/2): 11-15.
[15] MA B Y, TAN C, YU J K. Effects of heating temperature and addition amount of La2O3 on synthesis of ZrO2-SiC composite [J]. China’s Refractories, 2007, 16(suppl.1): 158-160. (in Chinese)
[16] CHEN Z Y. Chemical thermodynamics of refractories [M]. Beijing: Metallurgical Industry Press, 2005: 494-499. (in Chinese)
Foundation item: Project(50274021) supported by the National Natural Science Foundation of China and Baoshan Iron and Steel Co. Ltd.
Corresponding author: YU Jing-kun; Tel/Fax: +86-24-83681576; E-mail: jingkunyu@yahoo.com
(Edited by YUAN Sai-qian)