DOI:10.19476/j.ysxb.1004.0609.2017.10.25
铝酸钾溶液碳酸化分解的动力学
孙迎姣1,王 菊1,杜春华1,郑诗礼2,张 懿2
(1. 青岛农业大学 化学与药学院,青岛 266109;
2. 中国科学院 过程工程研究所,北京 100080)
摘 要:对铝酸钾溶液碳酸化分解过程进行研究,建立碳酸化分解过程的动力学模型。借鉴拜耳法晶种分解的动力学模型,对碳分动力学数据进行多元回归,得到动力学方程。结果表明:碳酸化分解的表观活化能为39.2708 kJ/mol,说明铝酸钾溶液碳酸化分解需要突破的壁垒小;瞬时晶种量对碳酸化分解过程的影响较小;相比铝酸钠溶液碳酸化分解,苛碱浓度对铝酸钾溶液分解的影响更大。铝酸钾溶液在40~80℃碳化分解,所得氢氧化铝为拜耳石型。
关键词:铝酸钾溶液;碳酸化分解;动力学模型;多元线性回归;表观活化能
文章编号:1004-0609(2017)-10-2164-06 中图分类号:TQ013.5 文献标志码:A
铝土矿为不可再生的铝资源,已成为美国等发达国家战略储备矿产之一。我国已探明的铝土矿总量只占世界储量的1.5%。近年来随着氧化铝产能的扩大,我国对铝土矿的进口依赖度大增,2014年进口3628万t,2015年进口5580万t左右。研究利用非铝土矿型含铝矿物生产氧化铝来缓解铝资源紧缺压力日渐显示出实用价值。我国具有丰富的非铝土矿型铝资源,仅钾长石资源保有量就超过一百亿t,可作为提铝、提钾、提硅的原料。将霞石、钾长石等含铝、钾矿物制备铝酸钾溶液,而后用二氧化碳碳酸化分解(简称碳 分)制备氢氧化铝、提取碳酸钾的技术有待深入研究。研究碳分动力学,可为探讨过程机理、优化碳分工艺、提高产品品质提供理论依据,为非铝土矿的实际开发应用提供技术支撑。且其相关研究未见文献报道。
目前,工业生产氧化铝几乎全属于碱法,采用NaOH或Na2CO3,而未采用KOH或K2CO3,致使国内外对铝酸钾溶液研究较少,且集中在K+对拜耳法铝酸钠溶液氢氧化铝成核、附聚等过程的影响方面[1-2]。铝酸钾溶液的基本体系为KOH-Al2O3-H2O体系;碳分过程中KOH转化为K2CO3,其基本体系为KOH- Al2O3-K2CO3-H2O 体系。DU等[3-5]对KOH-Al2O3- K2CO3-H2O体系及其子体系相平衡进行了大量的研究,为本研究奠定了基础。
目前,国内外对铝酸钠溶液晶种分解过程和碳分过程及其动力学研究较多[6-12]。碳分是联合法和烧结法中最重要的工序之一。对于碳分机理,李小斌等[12]和毕诗文[13]对各种观点进行了详细评价,但目前尚未有定论。在此,本文作者研究铝酸钾溶液碳分过程,可为铝酸钠溶液碳分研究提供参考。
鉴于铝酸钾溶液碳分过程的复杂性,本文作者进行了大量溶液结构、分解热力学研究,相关成果正陆续发表;并试图从动力学角度对该过程的机理进行了解。
本文作者认为,在碳酸化分解过程中,通入二氧化碳气体与氢氧化钾反应生成碳酸钾,溶液碱度降低,使溶液保持了较高的过饱和度,克服了溶液强稳定性的瓶颈,一旦产生微细晶核,就与晶种分解过程相似。因此,建立动力学模型可以借鉴铝酸钠溶液晶种分解的模型。
1 实验
1.1 实验材料及仪器
分析纯铝粉,纯度≥99%(中国天津市巴斯夫化工有限公司生产),优级纯氢氧化钾(天津博迪化工股份有限公司生产)。
智能恒温水浴(巩义市予华仪器有限责任公司生产),转子流量计LZB-4(西安市仪器仪表工业公司自动化仪表一厂生产)。
D8 Advance多晶粉末衍射仪(德国布鲁克AXS有限公司生产)。
1.2 实验装置
实验装置主要由反应系统和进气系统组成。反应系统包括特制的316L材质不锈钢桶反应器,控速机械搅拌,控温精度为±1 ℃的恒温水浴。进气系统采用LZB-4玻璃转子流量计控制CO2和N2的流量(CO2的通气速度80 L/h,N2的通气速度130 L/h),CO2和N2经自制混合器混合后进入反应器。
1.3 实验方法
在2 L的不锈钢桶中,少量多次地把定量铝粉加入到定量氢氧化钾溶液中,滤掉杂质,配成苛性比为1.5左右、氧化铝浓度120 g/L左右的铝酸钾溶液。反应过程中,在规定时间取样,经离心分离,取少量溶液,定容,滴定分析Al2O3、K2Ok、K2OT的浓度,并计算分解率η。
反应毕,将产品烘干,磨细,采用X射线衍射仪确定晶型,测定条件如下:扫描范围10°~90°,Cu Kα靶,管电压40 kV,管电流40 mA。
铝的测定采用EDTA-F-置换络合滴定法,先加入过量的EDTA,用0.025 mol/L的CuSO4标准溶液滴定,变成蓝紫色后加入氟化钠,然后以PAN为指示剂,再用CuSO4标准溶液滴定,根据第二次消耗CuSO4标准溶液的体积计算溶液中Al2O3的浓度。
总碱测定是以绿光酚酞为指示剂,用0.1 mol/L的HCl标准溶液滴定;苛碱测定是以绿光酚酞为指示剂,在待测液中加入适量的BaCl2溶液,再用HCl标准溶液滴定;总碱消耗的体积减去苛碱消耗的体积为碳碱消耗盐酸的体积。
2 结果与讨论
2.1 分解率与反应时间的关系
40~80 ℃下铝酸钾溶液碳分过程的分解率随时间的变化曲线如图1所示。该曲线近似呈S型,符合阿乌拉米方程 对曲线的要求[14],把阿乌拉米方程两边同时取对数,可化成
对曲线的要求[14],把阿乌拉米方程两边同时取对数,可化成
 ,对每条曲线的
,对每条曲线的 和
和 进行一元线性回归,就可得到每条曲线的分解率与反应时间关系的曲线方程。
进行一元线性回归,就可得到每条曲线的分解率与反应时间关系的曲线方程。
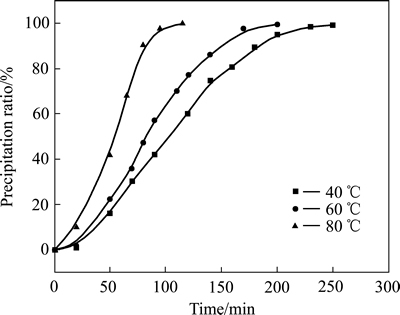
图1 分解率与时间的关系曲线
Fig. 1 Decomposition ratio verses reaction time curves
2.2 氢氧化铝的晶型
对40~80 ℃下所得样品进行X射线衍射分析,发现不同温度下未彻底碳酸化的产品为同一物相。图2所示为样品的XRD谱。由表1可看出,样品衍射图的强线d值与01-083-2256号鉴定为拜尔石型的PDF卡片吻合较好。由此可确定生成的氢氧化铝为拜耳石型。
2.3 碳酸化分解动力学模型的建立
李小斌等[15]通过计算吉布斯自由能,推测出铝酸钠溶液碳分过程因连续通入CO2,使得溶液的过饱和度不断升高而析出氢氧化铝,形成自动催化过程。从反应热力学角度来看,碳酸化分解过程与拜耳法晶种分解过程机理上存在很大的相似性。彭志宏等[16]认为在Al(OH)3的结晶和结晶长大机理上,碳酸化分解过程和晶种分解过程具有很多的相似点。李小斌等[12]依据CO2中和NaOH过程的反应时间明显小于A1(OH)3析出过程所需时间,推测出A1(OH)3析出过程是碳分过程的控制步骤。参考上述论述,结合本实验观察,认为铝酸钾溶液碳酸化分解的动力学模型可借鉴拜耳法晶种分解的动力学模型。
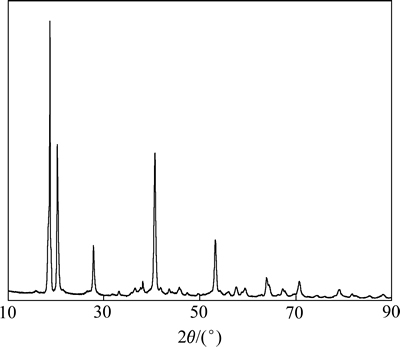
图2 所得样品的XRD谱
Fig. 2 XRD pattern of product
表1 所得氢氧化铝与PDF 01-083-2256的XRD强线对比
Table 1 Strong diffraction line comparison of product with PDF card (01-083-2256)
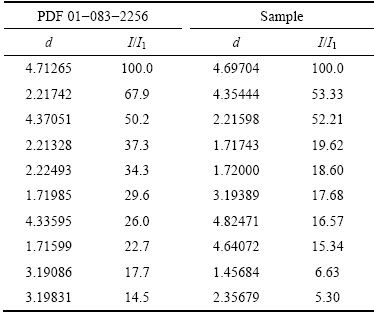
晶种分解动力学模型的一般形式[17-18]如下:
 (1)
(1)
式中:K为速率常数;A为溶液中Al2O3的瞬时浓度,g/L;Ae为溶液中A12O3的平衡浓度,g/L。
由于出发点不同,研究者们借鉴式(1)得出了很多形式相近的变形公式[8-10, 19-20],李小斌等[12]发现,WHITE等[19]提出的 动力学模型比较适宜铝酸钠溶液碳酸化分解过程。式中:G为反应速率;Nk为溶液中苛性碱的浓度。按Arrhenius公式构造动力学方程,具体形式如下:
动力学模型比较适宜铝酸钠溶液碳酸化分解过程。式中:G为反应速率;Nk为溶液中苛性碱的浓度。按Arrhenius公式构造动力学方程,具体形式如下:
 (2)
(2)
式中:W为单位体积溶液氧化铝析出量,g/L; 为分解时间,min;fr为瞬时晶种系数;T为分解温度,K;Ea为表观活化能,J/mol;K、n1、n2、n3为待定参数。
为分解时间,min;fr为瞬时晶种系数;T为分解温度,K;Ea为表观活化能,J/mol;K、n1、n2、n3为待定参数。
因为
 (3)
(3)
即 ,得
,得 。
。
所以
 (4)
(4)
将式(4)代入(2)中,得到
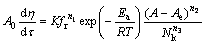 (5)
(5)
将式(5)的两边取对数得到
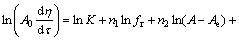
 (6)
(6)
式中:A0为分解原液中氧化铝的浓度,g/L;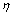 为溶液中氧化铝的分解率,%。
为溶液中氧化铝的分解率,%。
参照上述公式求取铝酸钾溶液碳分过程动力学参数。
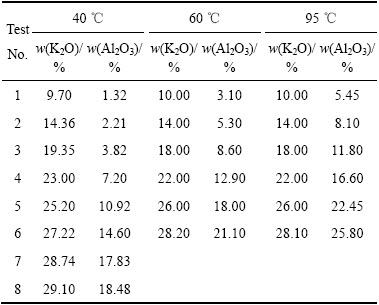
表2 40~95 ℃ K2O-Al2O3-H2O体系溶解度数据[3]
Table 2 Solubility of K2O-Al2O3-H2O system at 40-95 ℃[3]
2.4 碳酸化分解过程动力学模型参数的求解
把反应析出的氢氧化铝视为晶种,瞬时晶种定义为开始加入的晶种中所含氧化铝的量及反应过程中生成的氧化铝的量之和与溶液中还剩下的氧化铝的比值。由此可得
 (7)
(7)
式中:Kr为晶种系数。本研究中未添加晶种,则Kr=0。
WHITE等[20]提出的铝酸钠溶液中Al2O3平衡浓度的计算公式为
 (8)
(8)
研究发现,在低碱区K2O-Al2O3-H2O体系与Na2O-Al2O3-H2O体系溶解度曲线相似,对表2中40~95 ℃下K2O-Al2O3-H2O体系溶解度数据进行回归,得到铝酸钾溶液中Al2O3平衡浓度的计算公式:
 (9)
(9)
为求得 ,先对分解率与时间的关系曲线进行回归处理。曲线可用阿乌拉米方程表示,其形式为
,先对分解率与时间的关系曲线进行回归处理。曲线可用阿乌拉米方程表示,其形式为
 (10)
(10)
对式(10)两边求导,得
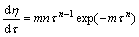 (11)
(11)
对于m、n,可通过对每条分解率与时间的关系曲线分别进行线性回归,得到不同温度下的经验方程如下所示,得到对应的m、n的值。
40 ℃下的经验方程:
 (12)
(12)
60 ℃下的经验方程:
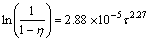 (13)
(13)
80 ℃下的经验方程:
 (14)
(14)
对实验数据按照式(6)进行多元线性回归,计算出待定参数K、n1、n2、n3、Ea的值。其中: 8.8279,n1=0.4437,n2=0.3075,n3=-1.0140,-Ea=-39270.8。相关系数r=0.993,观测值n=18,Ftest=155.1628,临界值F0.01(4,13)=5.21,说明回归效果高度显著。
8.8279,n1=0.4437,n2=0.3075,n3=-1.0140,-Ea=-39270.8。相关系数r=0.993,观测值n=18,Ftest=155.1628,临界值F0.01(4,13)=5.21,说明回归效果高度显著。
因此,铝酸钾溶液碳分过程的动力学模型可表示为:
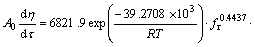
 (15)
(15)
2.5 对铝酸钾溶液碳分过程动力学模型的讨论
计算得到铝酸钾溶液碳分过程的表观活化能是39.2708 kJ/mol。众多研究者[21-23]获得的铝酸钠溶液晶种分解过程的表观活化能大约在49~83 kJ/mol,李小斌等[12]得到铝酸钠溶液碳分过程的表观活化能为75.115 kJ/mol。由此可见铝酸钾溶液碳酸化分解需要突破的壁垒小,反应较铝酸钠溶液体系更容易进行。可能是由于铝酸钾溶液较铝酸钠溶液的粘度小,碱性强,所以碳酸化分解的壁垒小,更容易反应。
模型中瞬时晶种的指数较小,可认为晶种量对碳酸化分解过程的影响较小。
与李小斌等[12]获得的铝酸钠溶液碳分动力学相比,铝酸钾溶液碳分动力学模型中苛碱的指数不同,可见苛碱浓度对铝酸钾溶液碳分的影响较大。
3 结论
1) 在碳酸化分解过程的动力学模型中,瞬时晶种项的指数较小,可见瞬时晶种量对碳分过程的影响较小。
2) 通过多元回归计算,得到铝酸钾溶液碳分过程的表观活化能为39.2708 kJ/mol,比铝酸钠溶液的碳分和晶种分解的表观活化能都小,说明反应所需突破的壁垒小,反应更容易进行。
3) 与铝酸钠溶液碳分相比,苛碱浓度对铝酸钾溶液分解的影响更大。
REFERENCES
[1] LI Jun, PRESTIDGE C A, JONAS A M. Secondary nucleation of gibbsite crystals from synthetic Bayer liquors: effect of alkali metal ions[J]. Journal of Crystal Growth, 2000, 219: 451-464.
[2] PRESTIDGE C A, IGOR A. Cation efects during aggregation and agglomeration of gibbsite particles under synthetic Bayer crystallisation conditions[J]. Journal of Crystal Growth, 2000, 209: 924-933.
[3] DU Chun-hua, ZHENG Shi-li, ZHANG Yi. Phase equilibria in the K2O-Al2O3-H2O system at 40 ℃[J]. Fluid Phase Equilibria, 2005, 238: 239-241.
[4] DU Chun-hua, ZHENG Shi-li, ZHANG Yi. Solubility of the system KOH-K2CrO4-Al2O3-H2O at 150 ℃ in a high alkali concentrated region[J]. Journal of Chemical & Engineering Data, 2012, 57(11): 2971-2974.
[5] DU Chun-hua, ZHENG Shi-li, ZHANG Yi. Solubility of the system KOH-K2CrO4-K2CO3-Al2O3-H2O at 150 ℃ in the high alkali concentrated region[J]. Journal of Chemical & Engineering Data, 2014, 59(8): 2423-2427.
[6] KING W R. Some studies of alumina trihydrate precipitation kinetics[J]. Light Metals, 1973, 1: 551-563.
[7] CRAMA W J, VISSER J. Modeling and computer simulation of alumina trihydrate precipitation[C]// Light Metals. Pennsylvania: TMS, 1994: 73-82.
[8] PEARSON T G. The chemical background to the aluminum industry[M]. London: Royal Institute of Chemistry Monograph, 1955: 50-55.
[9] SCOTT J. Extractive metallurgy of aluminum[M]. New York: Interscience Publishers, 1963: 203-218.
[10] AUDET D R, LAROCQUE J E. Development of model for precipitation of productivity of alumina hydrate precipitation[C]// Light Metals. Pennsylvania: Metallurgical Soc of AIME, 1989: 21-31.
[11] MISRA C. Solubility of aluminum trihydroxide hydrargillite in sodium hydroxide solution[J]. Chemistry and Industry, 1970, 20: 619-623.
[12] 李小斌, 陈 斌, 周秋生, 刘桂华, 彭志宏, 刘祥民. 铝酸钠溶液碳酸化分解动力学[J]. 中国有色金属学报, 2004, 14(5): 848-853.
LI Xiao-bin, CHEN Bin, ZHOU Qiu-sheng, LIU Gui-hua, PENG Zhi-hong, LIU Xiang-min. The kinetics of carbonation decomposition of sodium aluminate[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(5): 848-853.
[13] 毕诗文. 氧化铝生产工艺. 北京: 化学工业出版社, 2006.
BI Shi-wen. Theproductiontechnologyofthealumina. Beijing: Chemical Industry Press, 2006.
[14] 孙 康. 宏观反应动力学及其解析方法[M]. 北京: 冶金工业出版社, 1998: 46-47.
SUN Kang. Apparent reaction kinetics and its analytical methods[M]. Beijing: Metallurgical Industry Press, 1998: 46-47.
[15] 李小斌, 刘祥民, 苟中入, 彭志宏, 刘桂华, 周秋生, 丁安平, 李 明, 刘业翔. 铝酸钠溶液碳酸化分解的热力学[J]. 中国有色金属学报, 2003, 13(4): 1006-1007.
LI Xiao-bin, LIU Xiang-min, GOU Zhong-ru, PENG Zhi-hong, LIU Gui-hua, ZHOU Qiu-sheng, DING An-ping, LI Ming, LIU Ye-xiang. The thermodynamics of carbonation decomposition of sodium aluminate[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(4): 1006-1007.
[16] 彭志宏, 李小斌, 苟中入, 刘桂华, 周秋生, 丁安平, 李光柱, 李 明. 铝酸钠溶液碳酸化分解产品中的Na2O[J]. 中国有色金属学报, 2002, 12(6): 1285-1289.
PENG Zhi-hong, LI Xiao-bin, GOU Zhong-ru, LIU Gui-hua, ZHOU Qiu-sheng, DING An-ping, LI Guang-zhu, LI Ming. Impurity Na2O in carbonization precipitation from sodium aluminate solution with high Al2O3 concentration[J]. The Chinese Journal of Nonferrous Metals, 2002, 12(6): 1285-1289.
[17] HALFON A. Alumina trihydrate crystallization (part l): Secondary nucleation and growth rate kinetics[J]. The Canadian Journal of Chemical Engineering, 1976, 54(6): 160-167.
[18] YOUSRY L S. Dynamic simulation and control of the Bayer process: A review[J]. Industrial & Engineering Chemistry Research, 2001, 40(4): 1146-1156.
[19] WHITE E T, BATEMAN S H. The effect of caustic concentration on the growth rate of Al(OH)3 particles[J]. Light Metal, 1988(1): 157-162.
[20] MISRA C, WHITE E T. Kinetics of crystallization of aluminate trihydroxide from seeded caustic aluminate solution[J]. Chemical Engineering Progress Symposium Series, 1970, 110: 53-56.
[21] 谢雁丽, 吕子剑, 毕诗文, 赵 群, 王锡慧, 姜小凯, 杨毅宏. 铝酸钠溶液晶种分解[M]. 北京: 冶金工业出版社, 2003: 138-139.
XIE Yan-li,  Zi-jian, BI Shi-wen, ZHAO Qun, WANG Xi-hui, JIANG Xiao-kai, YANG Yi-hong. Seeded precipitation of sodium aluminate solution[M]. Beijing: Metallurgical Industry Press, 2003: 138-139.
Zi-jian, BI Shi-wen, ZHAO Qun, WANG Xi-hui, JIANG Xiao-kai, YANG Yi-hong. Seeded precipitation of sodium aluminate solution[M]. Beijing: Metallurgical Industry Press, 2003: 138-139.
[22] 方敬东, 吴素芳, 王樟茂. 铝酸钠溶液分解反应研究[J]. 高校化学工程学报,2002, 16(1): 33-36.
FANG Jing-dong, WU Su-fang, WANG Zhang-mao. The studies of precipitation reaction of sodium aluminate[J]. Journal of Chemical Engineering of Chinese Universities, 2002, 16(1): 33-36.
[23] SKOUFADIS C, PANIAS D, PASPALIARIS I. Kinetics of boehmite precipitation from supersaturated sodium aluminate solutions[J]. Hydrometallurgy, 2003, 68: 57-68.
Kinetics of carbonation decomposition of potassium aluminate solution
SUN Ying-jiao1, WANG Ju1, DU Chun-hua1, ZHENG Shi-li2, ZHANG Yi2
(1. College of Chemistry and Pharmacy, Qingdao Agricultural University, Qingdao 266109, China;
2. Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100080, China)
Abstract: The kinetics of carbonation decomposition of potassium aluminate solution was investigated and the kinetic model was established. Referencing seeded precipitation model, the kinetic equation was deduced through multiple linear regression according to experimental data. The equation shows that the apparent activation energy is 39.2708 kJ/mol, demonstrating that the carbonation decomposition reaction of potassium aluminate solution is easier than that of sodium aluminate solution. The instantaneous seed quantity has a small effect on the process of carbonation decomposition; the alkali concentration has more significant impact on the carbonation decomposition of potassium aluminate solution than that of sodium aluminate solution. XRD analysis demonstrates that the obtained products at 40-80 ℃ belong to Gibbsite Al(OH)3.
Key words: potassium aluminate solution; carbonation decomposition; kinetic model; multiple linear regression; apparent activation energy
Foundation item: Projects(21346010, 50874099, 51774261) supported by the National Natural Science Foundation of China
Received date: 2016-05-26; Accepted date: 2016-11-27
Corresponding author: DU Chun-hua; Tel: +86-532-88030522; E-mail: dch1218@163.com
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(21346010,50874099,51774261)
收稿日期:2016-05-26;修订日期:2016-11-27
通信作者:杜春华,副教授,博士;电话:0532-88030522;E-mail: dch1218@163.com