纳米二氧化锰的电化学电容性能
张 莹1,刘开宇1,张 伟1, 2,王洪恩1
(1. 中南大学 化学化工学院,湖南 长沙,410083;
2. 中船重工第七一二研究所,湖北 武汉,430064)
摘 要:以MnSO4和K2S2O8为原料,采用液相法制得MnO2并制成电化学电容器电极;采用X射线衍射和扫描电镜对产物进行结构形貌表征,采用恒流充放电、循环伏安、交流阻抗等方法对MnO2电化学电容器电化学性能进行表征。研究结果表明:产物为纳米棒聚集而形成的纳米α-MnO2。充放电曲线由于电极在0.53 V和0.36 V(vs. Hg/HgO)处发生氧化还原反应而发生了明显弯曲,这有利于比容量的提高;在10-3~104 Hz频率范围内,阻抗曲线在0.2 Hz以下出现“电荷饱和”,说明电极材料中储存的大部分电容量可得到利用,有效能量为 48.3 J/g,电极具有良好的倍率特征,其频率响应时间为12.5 s;在低频区电极过程由阻挡层扩散控制,比容量可达到151 F/g,但随着频率增加,比容量快速下降,100 Hz以后比容量开始趋于0。
关键词:纳米二氧化锰;电化学电容器;准电容
中图分类号:TM53;TM911 文献标识码:A 文章编号:1672-7207(2008)03-0469-05
Electrochemical-capacity of nanostructure manganese dioxide
ZHANG Ying1, LIU Kai-yu1, ZHANG Wei1, 2, WANG Hong-en1
( 1. School of Chemistry and Chemical Engineering, Changsha 410083, China;
2. No.712 Research Institute, China Shipbuilding Industry Corporation, Wuhan 430064, China)
Abstract: Using MnSO4 and K2S2O8 as reactants, MnO2 was synthesized using fluid phase method and assembled to the electrochemical capacitor electrode. XRD and SEM were used to investigate its structure and morphology, and galvanostastic charge-discharge, A.C. impendence, cyclic voltammetry were used to study the electrochemical performance of the as-prepared electrochemical capacitor. The results show that the product is pure nanostructure α-MnO2, assembled by plenty of nano-robs. The charge-discharge curves bend obviously, resulting from the redox reactions in 0.53 V and 0.33 V (vs. Hg/HgO) respectively, which is helpful to enhance the capacity. In the range of 10-3-104 Hz, “charge saturation” occurs below 0.2 Hz, showing that most capacitance in the electrode material can be made good use of, and the available power is 48.3 J/g. The rate capability of the electrode is favorable, with the characteristic response time 12.5 s. The electrode process is determined by the block-layer diffusion at low frequency, with a specific capacitance as high as 151 F/g. However, the capacity drops quickly along with the increase of frequency. Below 100 Hz, the capacitance approximates to 0.
Key words: nanostructure manganese oxide; electrochemical capacitor; pseudocapacitance
电化学电容器是介于传统电容器和化学电源之间的新兴储能器件,它比传统电容器具有更高的能量密度,比电池具有更高的功率密度,具有广阔的应用前景[1]。以RuO2 等贵重金属氧化物为电极材料的电化学电容器因具有比双电层电容器更高的比电容已应用于多个领域,但因成本昂贵限制了其应用[2]。一些廉价金属氧化物如NiO和MnO2等[3-4] 也具有氧化还原准电容,其中MnO2资源丰富,电化学性能好,已成为电化学电容器电极材料的研究热点[5-8]。人们对纳米MnO2的制备研究较多,如张宝宏等[9]用固相合成法制备纳米MnO2粉末,其在1 mol/L KOH溶液中比容量可达240 F/g,经5 000次循环,电极容量保持90%以上;张治安等[10]用化学共沉淀法制备纳米水合氧化锰,其比电容达到203.4 F/g。V. Subramanian等[11]采用水热法制备了结晶性良好的纳米MnO2,在电流密度为200 mA/g时获得的比容量为168 F/g;刘献明 等[12]采用液相法制备出结晶性能良好的超细MnO2,其容量可达150 F/g,并具有优越的循环稳定性能。在此,本文作者采用液相法制备纳米棒聚集而形成纯相纳米α-MnO2,以此为对称型电化学电容器电极材料,对其进行充放电、循环伏安与交流阻抗测试,对其电化学电容性能进行研究。
1 实 验
1.1 材料制备与表征
将物质的量相等的分析纯MnSO4和K2S2O8溶于一定量的去离子水中,加入适量硫酸调节溶液pH=1,将所得溶液于60 ℃保温22 h。反应完成后,将所得黑色沉淀抽滤,并用去离子水和无水乙醇反复洗涤以除去杂质离子。将所得产物于110 ℃干燥5 h,即得所需MnO2样品。
采用D-500型X射线衍射仪(德国Siemens)对样品进行XRD测试(Cu Kα靶材,石墨单色器,管电压为36 kV,管电流为36 mA, 扫描范围2θ为10?~70?,扫速为4 (?) /min,波长为0.154 18 nm)。采用X-650型扫描电子显微镜(日本理学)对样品进行形貌表征。
1.2 电极制备与电化学测试
将所制MnO2与乙炔黑、PTFE(质量分数为5%)按质量比75?15?10混合均匀,采用辊压法,以泡沫镍为集流体,将其压成厚度为0.3 mm的电极片,于120 ℃真空干燥至恒重(活性物质约54 mg)。将2片相同的电极用隔膜纸隔开,以6 mol/L KOH为电解液,组装成夹心式对称型电化学电容器。采用LANDCT2001A型电池测试系统(武汉金诺)在电压为0.8 V、电流密度为400 mA/g条件下对电化学电容器进行充放电测试,以Hg/HgO电极作参比电极,分别测试正、负极充放电时的工作电位范围,测试装置如图1所示;在三电极体系下(Hg/HgO电极为参比电极,2 cm×2 cm的铂电极为辅助电极,6 mol/L KOH溶液为电解液),采用CHI660电化学工作站(上海辰华)在15 mV/s扫描速度下对上述电极进行循环伏安测试,并在频率为10-3~104 Hz时对上述电极进行交流阻抗测试。
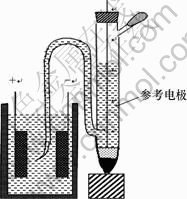
图1 电化学电容器测试装置
Fig.1 Testing system of electrochemical capacitor
2 结果与讨论
2.1 结构与形貌表征
图2所示为产物MnO2的XRD图谱,对照标准卡片(PDF 44-0141),其主要特征峰分别对应于α-MnO2晶体的(211),(400),(310)和(301)等晶面, 各特征峰的峰型较尖锐,强度较大,表明所得样品为四方晶系的α-MnO2。
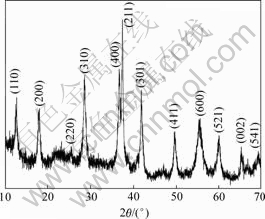
图2 所制备的MnO2 的XRD图谱
Fig.2 XRD pattern of as-prepared MnO2
图3所示为产物MnO2的SEM图谱。可以看出,所得MnO2呈直径为30~70 nm,长度约600 nm的纳米棒聚集而成的三维菊花状形貌。
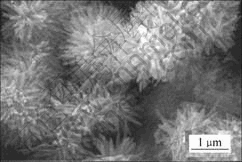
图3 MnO2的SEM图
Fig.3 SEM image of as-prepared MnO2
2.2 充放电测试
在电压为0.8 V、电流密度为400 mA/g时电化学电容器的充放电测试结果如图4所示。可见,正极充电曲线在0.53 V附近、放电曲线在0.36 V附近出现了明显的弯曲现象,而负极未出现此现象。

1—正极电位E;2—负极电压E;3—电容器电压V
图4 电化学电容器充放电曲线
Fig.4 Charge-discharge curves of electrochemical capacitor and electrode
图5所示为扫描速度为15 mV/s,电压为-0.6~ 0.3 V时电极的循环伏安曲线。可以看出,在0.36 V处电极发生了明显的还原反应,而在0.53 V处发生了对应的氧化反应,这也说明超级电容器及正极充放电曲线发生弯曲现象是发生了氧化还原反应所致;氧化还原反应的存在有利于电容器容量的提高[13]。
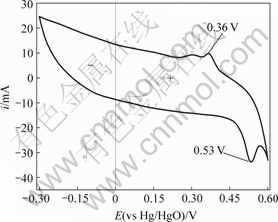
图5 电极的循环伏安曲线
Fig.5 CV curves of electrode
2.3 交流阻抗分析
图6所示为电极的复平面阻抗图。其中:Z′为阻抗实部;Z″为阻抗虚部。根据频率及阻抗曲线各段特征,可将阻抗曲线分成高频段、中频段和低频段。由图6中局部放大图可见,电极高频段的阻抗曲线表现为不完整半圆,而在中频段表现为1条倾角为45?的直线,这是多孔电极阻抗曲线的典型特征。在低频段,电极在频率255 mHz以下出现“电荷饱和”[14](即在此频率下交流阻抗曲线开始垂直于Z′轴,意味着在此频率以下,电容器的大部分电容量均可以得到利用)。

图6 电极复平面阻抗图
Fig.6 Nyquist plot of electrode (10-3~104 Hz)
图7所示为电极的-φ-lg f曲线。在测试频率下限,即lg f ≈-2.5时,|φ|为78?,随着频率的升高,|φ|减少很快,在lg f ≈1.69时变为6?,然后在lg f ≈2.33处出现1个相角的峰值,这对应于高频区发生的电极反应。并且,在10-3~104 Hz范围内,相位角均小于理想电容器的相位角90?。
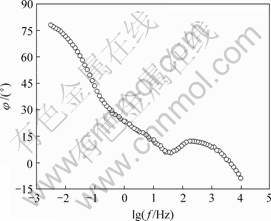
图7 电极-φ-lg f图
Fig.7 -φ-lg f diagram of electrode
在图6所示的低频区,近似垂直于Z′轴的直线说明此时电极过程为扩散控制,作电极tan φ-f图,如图8所示。可见,在低频区表现为阻挡层扩散控制特征[15]。
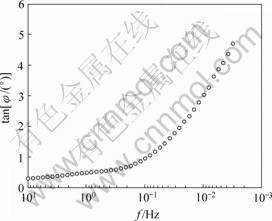
图8 电极tan φ-f曲线
Fig.8 tan φ-f curve of electrode
图9所示为电极比容量与频率的关系曲线。从图9可得出某一特定频率下电极材料的比电容,计算公式为[16]:
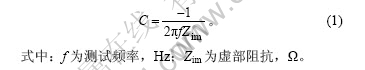
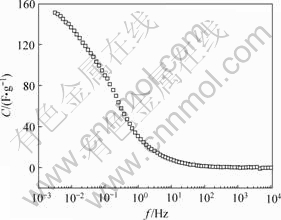
图9 电极比容量与频率的关系
Fig.9 Relationship between capacitance of electrode and frequency
由图9可见,在测试频率范围内,低频下电解液中的水合离子更容易进入材料的孔隙结构,电极比容量达到151 F/g;随着频率的增加,容量快速下降,在100 Hz以后,电极比容量开始趋于0,说明高频下水合离子均难以进入电极的孔隙中。作电极的lg Z′-lg f曲线和lg Z″-lg f曲线,如图10所示。根据文献[17]中提出的用交流阻抗表征电极倍率特征的方法,通过图10上特征频率f0(该点对应的相角为-45?,阻抗的实部和虚部相等)的倒数来计算反应时间t0。算得电极反应时间12.5 s,并且,利用下式可以计算电极的有效能量E0:

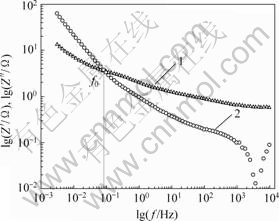
1—lg Z′; 2—lg Z″
图10 电极阻抗图
Fig.10 Impedance plots of electrode
3 结 论
a. 以MnSO4和K2S2O8为原料,采用液相法制得纳米棒聚集而形成的菊花状形貌纳米α-MnO2。
b. 恒流充放电及循环伏安测试结果表明,对称型纳米α-MnO2电化学电容器充放电曲线由于正极在0.53 V和0.36 V(vs. Hg/HgO)处发生氧化还原反应而发生了明显弯曲,这有利于电极比容量的提高。
c. 交流阻抗测试结果表明,在10-3~104 Hz频率范围内,阻抗曲线在0.2 Hz以下出现“电荷饱和”现象,说明电极材料中储存的大部分电容量可得到利用,有效能量为 48.3 J/g;电极具有良好的倍率特征,其频率响应时间为12.5 s;在低频区,电极过程由阻挡层扩散控制,比容量可达到151 F/g,但随着频率增加,比容量快速下降,100 Hz以后比容量开始趋于0。
参考文献:
[1] Burke A. Ultracapacitors: Why, how, and where is the technology[J]. Journal of Power Sources, 2000, 91: 37-50.
[2] 甘卫平, 黎小辉, 欧定斌, 等. 退火温度对钽基RuO2·nH2O电沉积薄膜电容性能的影响[J]. 中南大学学报:自然科学版, 2006, 37(4): 660-664.
GAN Wei-ping, LI Xiao-hui, OU Ding-bin, et al. Effect of annealing temperature on capacitance of ruthenium oxide films deposited on tantalum substrate[J]. Journal of Central South University: Science and Technology, 2006, 37(4): 660-664.
[3] Venkat S, John W W. An electrochemical route for making porous nickel oxide electrochemical capacitors[J]. Journal of Electrochemical Society, 1997, 144(8): L210-L213.
[4] YUAN Liang-jie, LI Zi-cheng, SUN Ju-teng, et al. Synthesis and characterization of activated MnO2[J]. Materials Letters, 2003, 57(13/14): 1945-1948.
[5] 夏 熙, 李 娟, 李清文. 纳米MnO2的固相合成及其电化学性质研究(II)——纳米γ-MnO2的电化学性能[J]. 高等学校化学学报, 1999, 20(10): 1584-1588.
XIA Xi, LI Juan, LI Qing-wen. Synthesis and electrochemical properties of nanophase MnO2 by solid phase reaction (II): Electrochemical properties of nanophase γ-MnO2[J]. Chemical Journal of Chinese Universities, 1999, 20(10): 1584-2588.
[6] 马淳安, 楼颖伟, 赵峰鸣, 等. 纳米MnO2的制备及电化学性能研究[J]. 中国有色金属学报, 2004, 14(10): 1736-1740.
MA Chun-an, LOU Ying-wei, ZHAO Feng-ming, et al. Synthesis and characterization of nano-size manganese dioxide[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(10): 1736-1740.
[7] 努尔买买提, 夏 熙. 纳米MnO2的制备及其性能研究[J]. 无机材料学报, 2000, 15(5): 802-806.
Nurmaimaiti, XIA Xi. Synthesis and performance of nanophase α-MnO2[J]. Journal of Inorganic Materials, 2000, 15(5): 802-806.
[8] 冯杨柳, 张密林, 陈 野, 等. 无机盐水溶液反应合成 MnO2纳米粉体及其电容特性[J]. 硅酸盐学报, 2005, 33(3): 318-322.
FENG Yang-liu, ZHANG Mi-lin, CHEN Ye, et al. Inorganic salt water solution reaction synthesis and capacitor characteristics of nano-MnO2 powder[J]. Journal of the Chinese Ceramic Society, 2005, 33(3): 318-322.
[9] 张宝宏, 张 娜. 纳米MnO2 超级电容器的研究[J]. 物理化学学报, 2003,19(3): 286-288.
ZHANG Bao-hong, ZHANG Na. Research on nanophase MnO2 for electrochemical supercapacitor[J]. Acta Phys Chim Sin, 2003, 19(3): 286-288.
[10] 张治安, 杨邦朝, 邓梅根, 等. 超级电容器纳米氧化锰电极材料的合成与表征[J]. 化学学报, 2004, 62(12): 1617-1620.
ZHANG Zhi-an, YANG Bang-chao, DENG Mei-gen, et al. Synthesis and sharacterization of nanostructured MnO2 for supercapacitor[J]. Acta Chmica Sinica, 2004, 62(12): 1617-1620.
[11] Subramanian V, Zhu H W, Robert V, et al. Hydrothermal synthesis and pseudo-capacitance properties of MnO2 nanostructures[J]. J Phys Chem B, 2005, 109: 20207-20214.
[12] 刘献明, 张校刚. 电化学电容器电极材料超细MnO2的制备及表征[J]. 化学研究与应用, 2003, 15(4): 515-517.
LIU Xian-ming, ZHANG Xiao-gang. Studies on ultrafine MnO2 as electrode material of electrochemical capacitor[J]. Chemical Research and Application, 2003, 15(4): 515-517.
[13] LIU Kai-yu, ZHANG Ying, ZHANG Wei, et al. Charge-discharge process of MnO2 supercapacitor[J]. Transactions of Nonferrous Metals Society of China, 2007, 17(3): 649-653.
[14] 梁 逵, 陈 艾, 冯哲圣, 等. 碳纳米管电极超大容量离子电容器交流阻抗特性[J]. 物理化学学报, 2002, 18(4): 381-384.
LIANG Kui, CHEN Ai, FENG Zhe-sheng, et al. A.C. impedance analysis of supercapacitors utilizing carbon nanotube electrodes[J]. Acta Phys Chim Sin, 2002, 18(4): 381-384.
[15] 曹楚南, 张鉴清. 电化学阻抗谱导论[M]. 北京: 科学出版社, 2002.
CAO Chu-nan, ZHANG Jian-qing. An introduction to electrochemical impedance spectroscopy[M]. Beijing: Science Press, 2002.
[16] 邢 伟, 刘欣梅, 白 鹏, 等. 新型有序炭纳米棒阵列的合成及在电化学电容器中的应用[J]. 中国科学: E辑, 2006, 36(7): 741-750.
XING Wei, LIU Xin-mei, BAI Peng, et al. Synthesize of a novel order carbon nanotube array and its application in supercapacitor[J]. Science in China: Series E, 2006, 36(7): 741-750.
[17] Lufrano F, Staiti P, Minutoli M. Evaluation of nafion based double layer capacitors by electrochemical impedance spectroscopy[J]. Journal of Power Sources, 2003, 124: 314-320.
收稿日期:2007-08-10;修回日期:2007-10-21
基金项目:国家自然科学基金资助项目(50772133)
通信作者:刘开宇(1967-),男,湖南长沙人,博士,副教授,从事致密能源(化学电源与超级电容器)及相关材料的研究;电话:13548590751;E-mail: kaiyuliu@263.net