文章编号:1004-0609(2014)04-1070-06
锌冶炼含镉烟尘制备高纯镉粉的新工艺
刘 远,郑雅杰,孙召明
(中南大学 冶金与环境学院,长沙 410083)
摘 要:采用硫酸浸出锌冶炼含镉烟尘,得到含镉硫酸浸出液,在硫酸镉浸出液中加入双氧水和FeCl3溶液,用NaOH溶液调节pH值后过滤,将滤液加入NaOH溶液中得到Cd(OH)2粉体,采用氢气还原得到镉粉。结果表明:当硫酸浓度为110 g/L、反应温度为65 ℃时,镉浸出率达99.63%。双氧水用量为理论量10倍、n(Fe)/n(As)为3:1、pH=5.5时,砷的去除率达99.5%,得到净化的硫酸镉溶液。将硫酸镉溶液以缓慢加料方式加入浓度为2 mol/L的NaOH溶液中,反应温度为25 ℃,控制终点pH=10,过滤洗涤得到粒径为10~20 μm的Cd(OH)2粉体,采用氢气还原Cd(OH)2粉体,在反应温度为310 ℃、反应时间120 min、氢气流量40 L/h时,得到平均粒径为49.61 μm的球形镉粉。
关键词:焙烧烟尘;除杂;化学沉淀法;氢气还原;镉粉;锌冶炼
中图分类号:TF114.1 文献标志码:A
New technology of high purity Cd powder prepared from roasting dust of zinc smelting
LIU Yuan, ZHENG Ya-jie, SUN Zhao-ming
(School of Metallurgy and Environment, Central South University, Changsha 410083, China)
Abstract: The CdSO4 solution was prepared by leaching roasting dust of zinc smelting with sulfuric acid, then, the CdSO4 solution was purified by adding hydrogen peroxide and ferric chloride and adjusting pH with sodium hydroxide. Cd(OH)2 powder was prepared after the purified CdSO4 solution mixed with NaOH solution, then, reduced with hydrogen. The results show that the leaching rate of Cd reaches 99.63% under the condition of H2SO4 concentration of 110 g/L, reaction temperature of 65 ℃. Under the condition that n(Fe)/n(As) is 3:1 and pH is 5.5, the removal rate of As can be higher than 99.5%. The obtained Cd(OH)2 particle size is 10-20 μm when the reaction temperature is 25 ℃, pH value of the solution is 10. High purified Cd powders were prepared from Cd(OH)2 particles reduced by hydrogen. The optimum process conditions are as follows: reaction temperature is 310 ℃, reaction time is 120 min and hydrogen flow is 40 L/h. The average particles size of spherical Cd powder is about 49.61 μm.
Key words: roasting dust; impurity; chemical precipitation; hydrogen reduction; Cd powder; zinc smelting
镉因其独特的物理和化学性质,使镍镉电池以高功率系数、长循环寿命、快速充电能力在军用无人机、通信电台和夜视仪等电子产品中得到广泛应用[1-2],近年来,镉在光电半导体材料[3-5]方面的应用前景使高纯镉粉的制备研究受到广泛重视。 镉资源在自然界中存在于锌矿、铅锌矿和铜铅锌矿中,在浮选时大部分进入锌精矿,在焙烧过程中富集于烟尘和高镉锌中[6-7]。我国火法炼锌厂和铜铅冶炼厂含镉物料经焙烧脱除砷、锑等杂质,得到浸出性能良好的焙砂,再用硫酸浸出[8-11],浸出液经氧化水解脱除铁、砷等杂质,净化后含镉溶液用锌粉置换得到海绵镉[12],在铸铁锅内于熔融烧碱保护下,铸成粗镉锭。此法存在工艺流程繁琐、生产周期长、能耗高且锌粉消耗量大,而回收的镉纯度低,限制了镉的生产和应用[13]。精镉是由粗镉经电解精炼法[14]或蒸馏法[15-16]进一步提纯得到。电解精炼法生产精镉其电流效率低、电耗大、周期长、成本高、电解沉积物不稳定,仍需进一步火法精炼[17]。蒸馏法采用精馏塔分离高沸点杂质制备粗镉,再进行碱性精炼得到精镉,其设备简单,生产控制方便,在我国锌冶炼厂普遍采用,但其精镉产出率低,镉与残渣分离不彻底,存在二次污染和镉中毒隐患[18]。精镉制备镉粉的方法有等离子还原法[19]、电化学法[20-21]、蒸发法[22]等。这些方法生产镉粉制备工艺周期长、温度高,镉粒在此过程中易长大,通常得不到超细镉粉。本文作者以锌冶炼含镉烟尘为原料,采用硫酸浸出,氧化中和除杂,氢氧化钠沉淀,氢气还原氢氧化镉制备球形高纯镉粉,与其他制备高纯金属镉方法相比,该工艺流程简单、环境友好、产品纯度高,且直接得到微米级球形镉粉,其市场应用广泛。
1 实验
1.1 实验原料
以河南某公司锌焙烧烟尘为原料,其主要元素含量如表1所列。硫酸(AR)、H2O2(AR)、FeCl3(AR)、氢氧化钠(AR)、氢气(高纯,w(H2)>99.99%)。
表1 锌焙烧烟尘主要元素含量
Table 1 Main chemical components of roasting dust (mass fraction, %)

1.2 实验步骤
浸出:将去离子水与硫酸按比例配好置于三口烧瓶中,启动加热套加热至所需温度,在搅拌下将锌焙烧烟尘缓慢加入,反应一定时间后过滤,得到硫酸浸出液。
除杂:在浸出液中加入H2O2使As(Ⅲ)氧化为As(Ⅴ),按照一定的n(Fe)/n(As)加入FeCl3溶液,在搅拌下将NaOH加入到浸出液中,调节溶液pH,使As、Fe、Cu和Pb沉淀除去,得到硫酸浸出净化液,即硫酸镉溶液。
氢氧化镉粉体的制备:以净化液为原料配制0.5mol/L的硫酸镉溶液,在连续搅拌下向硫酸镉溶液中加入NaOH溶液,发生如下反应:
CdSO4+NaOH=Cd(OH)2↓+Na2SO 4 (1)
在室温下反应一段时间后,将沉淀物过滤,多次洗涤,在75 ℃下干燥10 h,得到氢氧化镉微粉。
镉粉的制备:将氢氧化镉粉体置于程控管式炉中,通入N2保护,控制一定温度,在氢气中还原得到镉粉。锌冶炼含镉烟尘制备高纯镉粉流程如图1所示。

图1 锌冶炼烟尘制备高纯球形镉粉工艺流程图
Fig. 1 Flow chart for preparation of spherical Cd powder from roasting dust of zinc smelting
1.3 分析与检测
采用电感耦合等离子光谱仪(Intrepid Ⅱ XPS)分析物料中杂质含量;采用日本Rint-2000型X射线衍射仪分析样品的物相组成;采用扫描电镜(SEM、JEOL、JSM-5600LV)对样品的形貌进行分析表征;采用激光粒度分析仪(Mastersizer 2000)对产物的粒度进行分析表征。
2 结果与讨论
2.1 锌焙烧烟尘硫酸浸出除杂
实验采用硫酸浸出含镉烟尘,反应温度为65 ℃,液固比为6:1,硫酸浓度为110 g/L,反应时间为30 min。在此条件下硫酸浸出滤液中各元素含量如表2所列,镉的浸出率达到99.63%。
表2 锌焙烧烟尘硫酸浸出液中元素的含量
Table 2 Elements content in sulfuric acid leaching solution of zinc roasting dust (mg/L)
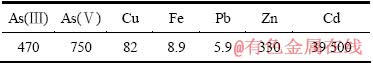
氧化镉溶于硫酸的反应方程式为
CdO+2H+=Cd2++H2O (2)
当氧化镉溶解反应达到平衡时,其平衡常数如下:
 (3)
(3)
在标准状态下,反应的标准吉布斯自由能变化为
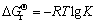 (4)
(4)
根据热力学数据表可知,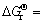 -86.10 kJ/mol,则标准状态下反应的平衡常数为
-86.10 kJ/mol,则标准状态下反应的平衡常数为
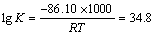
K = 1034.8 (5)
热力学计算表明,氧化镉在酸性溶液中反应趋势很大,反应在达到平衡状态时,在较小的H+浓度下,可以存在很高浓度的Cd2+。铅主要以硫酸铅存在,在硫酸介质中不溶解,大部分进入渣中,As、Zn、Fe、Cu主要以氧化物存在,在酸性条件下易溶解进入溶液,为得到纯净的硫酸镉溶液,需深度除杂。
2.2 锌焙烧烟尘硫酸浸出液除杂
在室温下加入理论量10倍的30%双氧水,反应5 min后加入FeCl3溶液,溶液中n(Fe)/n(As)=3,加入NaOH调节溶液pH为5.5,反应时间30 min,过滤得硫酸镉滤液,砷的脱除率达99.5%。滤液中杂质含量如表3所列。
表3 硫酸浸出净化液中杂质含量
Table 3 Impurities of purification sulfuric acid leaching solution (mg/L)
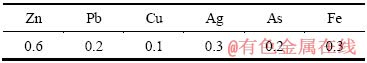
将浸出液中和到一定的pH,则发生以下水解反应:
Men++nOH-=Me(OH)n↓ (6)
水解平衡时:
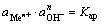 (7)
(7)
而
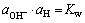 (8)
(8)
所以
 (9)
(9)
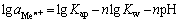 (10)
(10)
式中:Kap为水溶液中Men+与OH-的活度积;Kw为水的离子积,常温下近似等于1×10-14。氢氧化物沉淀时,杂质离子的平衡pH值如表4所列[23]。
表4 25 ℃时杂质离子的平衡pH值[23]
Table 4 Equilibrium pH value of impurity ions at 25 ℃[23]

在pH=5.5时,大部分杂质元素水解沉淀而除去,在砷酸盐沉淀过程中,发生如下反应:
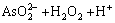 =
= (11)
(11)
H3AsO4+Fe3+=FeAsO4↓+3H+ (12)
对水解沉淀而言,同一变价金属的高价离子比低价离子容易水解,改变其价态可以改变其在水解过程的行为,因此加H2O2使As(Ⅲ)氧化为As(Ⅴ),促使溶液中砷离子完全沉淀,FeAsO4的溶度积为5.7×10-21,Fe(OH)3的溶度积为4×10-38,调节溶液pH可实现砷和铁同时去除,而Fe3+水解生成的Fe(OH)3胶体沉淀,也能吸附部分杂质元素。
2.3 氢氧化镉粉体的制备
慢速加料法是在反应开始前,将0.5 mol/L的硫酸镉溶液以5 mL/min的流量通过蠕动泵加入2 mol/L的NaOH溶液中,搅拌速度为400 r/min,控制反应终点pH=10,反应结束后,过滤、洗涤得到氢氧化镉粉体。快速加料法是将0.5 mol/L硫酸镉溶液和2 mol/L氢氧化钠溶液快速混合加入反应器中,之后操作与慢速加料法相同。图2(a)所示为快速混料方式得到的氢氧化镉粉体形貌。图2(b)所示为缓慢加料方式得到的氢氧化镉粉体形貌。
由图2(a)可以看出,氢氧化镉呈絮状,分散性差,团聚严重,使一部分颗粒粒径很大,另一部分颗粒粒径又很小。从图2(b)可以看出,缓慢加料法得到的氢氧化镉分散性好,粒径分布集中,团聚不严重。表明通过缓慢加料法得到的氢氧化镉具有更好的形貌,因为在缓慢加料过程中,反应物的加入速度很慢,反应产物过饱和度较低,不利于晶粒的生长和晶核的形成,整个反应过程中形成的晶核数量较少[23]。
慢速加料法得到Cd(OH)2的XRD谱如图3所示。由图3可看出,通过化学沉淀法制备的Cd(OH)2为六方晶系,并且从谱图上观察不到杂相峰存在,产物为Cd(OH)2晶体。
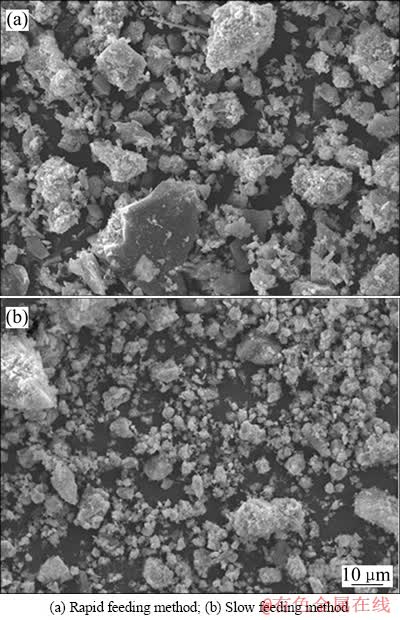
图2 不同加料方法得到的氢氧化镉SEM像
Fig. 2 SEM images of Cd(OH)2 prepared by different feeding rates

图3 氢氧化钠沉淀制备Cd(OH)2的XRD谱
Fig. 3 XRD patterns of Cd(OH)2 prepared by sodium hydroxide precipitation
2.4 氢气还原氢氧化镉制备高纯镉粉
2.4.1 还原温度对还原产物及其形貌的影响
称取烘干的Cd(OH)2粉末置于程控管式炉中,通入N2赶走空气后通入H2,在一定温度下反应时间为120 min。不同反应温度时,产物XRD实验结果如图4所示。

图4 不同温度还原产物的XRD谱
Fig. 4 XRD patterns of products at different reduction temperatures
由图4可知,产物的衍射峰尖锐,随着温度的升高,Cd粉衍射峰逐渐变窄,且峰强逐渐增强,Cd晶粒沿某一晶面高度生长,结晶从无序趋向有序,温度的升高使晶粒长大。在温度低于310 ℃时,除含有主物相Cd(密排六方结构)外,产物中混有粒径不同的呈现黄灰色的CdO,CdO的产生是由于还原温度未达到Cd粉的还原温度,因此,一部分CdO尚未完全反应还含有CdO相,说明在还原温度比较低的时候Cd(OH)2前驱体粉一部分转换为CdO,且并未完全还原。在310 ℃以上还原得到的Cd粉纯度高,在温度为310 ℃和340 ℃时, CdO相消失,Cd(OH)2粉末完全还原,产物为纯净的Cd粉。当还原温度超过镉的熔点后,镉粉熔化长大成镉粒,粒径显著增加,为了得到粒径均匀的镉粉,采用还原温度应控制在300~310 ℃。
图5所示为不同还原温度得到产物的SEM像。由图5可知,氢气还原Cd(OH)2得到的产物呈球形,表面光滑,温度310 ℃比340 ℃时得到的颗粒细小,无团聚现象。
对还原温度为310和340 ℃的样品进行粒度分析,当还原温度为310和340 ℃时,镉粉平均粒径分别为49.61和76.09 μm,随着还原温度的升高,分布在粗粒区的颗粒增多,说明温度升高促进还原产物Cd晶粒的长大,与SEM像结果一致。
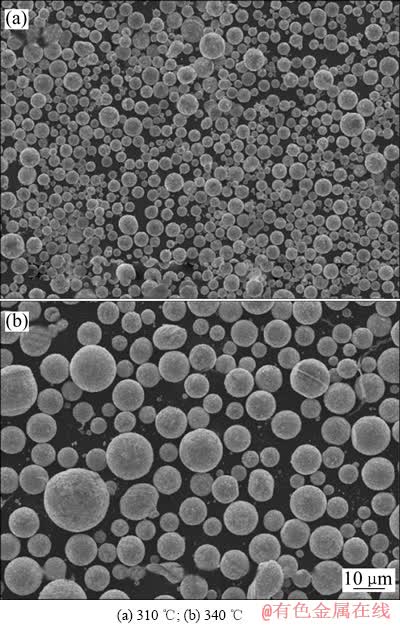
图5 不同还原温度产物的SEM像
Fig. 5 SEM images of products at different reduction temperatures
氢气还原Cd(OH)2粉末的过程就是Cd晶粒形核、长大、生长的过程。在还原过程中,先形成Cd晶核,随着还原的进行,新核不断生成,旧核不断长大,尺寸小的晶粒具有的应变能比尺寸大的晶粒的应变能大得多,为了尽可能地减小体系的总自由能而使结构趋于稳定,尺寸小的晶粒就自发合并成尺寸大的晶粒,在外界提供热量的情况下,界面附近的晶核发生扩散生长而使晶粒长大,当反应温度过低时,提供晶核扩散生长的能量不足,不能将其完全还原,产物中存在CdO物相,因此,需要升高还原温度使反应完全,但是随着反应温度的升高,晶粒的生长和镉的融化使其形成较大的颗粒,为了减小产物粒径,应严格控制还原温度。
2.4.2 还原时间对产物及粒度的影响
在其他条件不变,当还原温度为310 ℃时,还原时间对产物物相的影响如图6所示。从图6可知,当还原时间小于120 min时,还原产物中除了含有Cd外,还含有CdO相,说明CdO未还原完全;当还原时间大于120 min时,各还原产物的XRD谱均显示出单一的密排六方型Cd的衍射峰,且峰形尖锐。还原时间对制备纯净的Cd粉有较大的影响,随着还原时间的增加,烧结的作用越来越明显,其衍射峰高增加,峰宽变窄,镉粉的结晶度更好。
激光粒度仪分析表明,还原时间为60、120和180 min时产物平均粒径分别为38.76、49.61和73.25 μm。在一定的还原温度下,晶粒的尺寸符合Dt=ct1/2,即晶粒的平均直径与时间的平方根成正比,产物的平均尺寸随着反应时间的增加而增加,由晶粒聚集的颗粒也增大,随着还原时间的延长,细颗粒发生团聚,粗粒区的颗粒逐渐增加,镉粉的粒径明显变大,在保证镉粉质量的同时应尽量减少反应时间控制镉粉粒径。
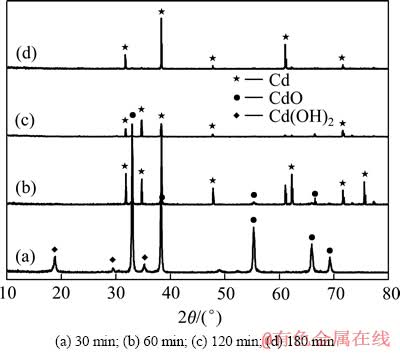
图6 不同还原时间产物的XRD谱
Fig. 6 XRD patterns of products at different reduction times
3 结论
1) 以锌冶炼含镉烟尘为原料,采用硫酸浸出得到含镉浸出液,加入理论量10倍双氧水,n(Fe)/n(As)=3,调节pH=5.5,过滤得到净化的硫酸镉溶液。将0.5 mol/L的硫酸镉溶液以5 mL/min的缓慢加料速度加入2 mol/L的NaOH溶液,终点pH=10,过滤洗涤烘干,得到平均粒径为10.56 μm的氢氧化镉粉体。
2) 采用H2还原Cd(OH)2前驱体制备高纯镉粉,还原温度和还原时间对产物结构和粒径影响较大,在还原条件为还原温度310 ℃,还原时间120 min,氢气流量40 L/h时,成功制备平均粒径为49.61 μm的球形镉粉。
REFERENCES
[1] ZHU Jian-xin, LI Jin-hui, NIE Yong-feng, YU Bo. Recovery of cadmium by high temperature vacuum evaporation from Ni-Cd batteries[J]. Transactions of Nonferrous Metals Society of China, 2003, 13(2): 254-257.
[2] 张 金, 王伯雄, 叶丽娜. 便携式军用镍镉电池智能检测与充电装置设计与实现[J]. 电子测量与仪器学报, 2010, 24(4): 359-364.
ZHANG Jin, WANG Bo-xiong, YE Li-na. Design and development of portable intelligent testing and charging equipment for nickel-cadmium battery[J]. Journal of Electronic Measurement and Instrument, 2010, 24(4): 359-364.
[3] HU Bao-yun, JING Zhen-zi, HUANG Jian-feng, YUN Jun. Synthesis of hierarchical hollow spherical CdS nanostructures by microwave hydrothermal process[J]. Transaction of Nonferrous Metals Society of China, 2012, 22(S1): s89-s94.
[4] 曹维良, 张凯华, 张敬畅. 纳米硫化镉制备条件对光催化活性的影响[J]. 北京化工大学学报, 2004, 31(1): 66-70.
CAO Wei-liang, ZHANG Kai-hua, ZHANG Jing-chang. Effects of conditions for preparation of nano-CdS on photocatalysis activity[J]. Journal of Beijing University of Chemical Technology, 2004, 31(1): 66-70.
[5] WANG Tao, JIE Wan-qi, XU Ya-dong, ZHA Gang-qiang, FU Li. Characterization of CdZnTe crystal grown by bottom-seeded Bridgman and Bridgman accelerated crucible rotation techniques[J]. Transactions of Nonferrous Metals Society of China, 2009, 19(S3): s622-s625.
[6] SADEGH S M, BAFGHI M S, MORADKHANI D, OJAGHI I M. A review on hydrometallurgical extraction and recovery of cadmium from various resources[J]. Minerals Engineering, 2007, 20: 211-220.
[7] 何 静, 唐谟堂, 刘 维. 氨法浸出提镉新工艺[J]. 化工学报, 2006, 57(7): 1727-1731.
HE Jing, TANG Mo-tang, LIU Wei. New process for extracting cadmium by ammonium-ammonia method[J]. Journal of Chemical Industry and Engineering, 2006, 57(7): 1727-1731.
[8] 成应向, 刘喜珍, 漆 燕, 王强强, 许友泽, 钟振宇. 有色冶炼铜镉渣中镉的提取工艺研究[J]. 环境工程, 2012, 30: 331-334.
CHENG Ying-xiang, LIU Xi-zhen, QI Yan, WANG Qiang-qiang, XU You-ze, ZHONG Zhen-yu. Process research of extracting cadmium from non-ferrous copper and cadmium slag[J]. Environmental Engineering, 2012, 30: 331-334.
[9] 齐美富, 乐红燕, 方小刚. 次氧化锌烟尘中镉的硫酸浸出动力学研究[J]. 能源与环境, 2009(5): 14-15.
QI Mei-fu, LE Hong-yan, FANG Xiao-gang. Study of cadmium sulfuric acid leaching kinetics from zinc oxide dust[J]. Energy and Environment, 2009(5): 14-15.
[10] WANG De-quan, JIANG Lan, LIN Mao-shen. Treatment of cadmium dust with two-stage leaching process[J]. Transactions of Nonferrous Metals Society of China, 2000, 20(2): 267-269.
[11] JHA M K, KUMAR V, SINGH R J. Review of the hydrometallurgical recovery of zinc from industrial wastes[J]. Resources, Conservation and Recycling, 2001, 33: 1-22.
[12] 范兴祥, 杨 卜. 某含锌镍镉烟尘元素分离及其产品制备工艺研究[J]. 矿产综合利用, 2009(4): 14-15.
FAN Xing-xiang, YANG Bo. Research on the technologies for separating valuable elements from the fly ash bearing Zn, Ni and Cd and preparing relative products[J]. Multipurpose Utilization of Mineral Resources, 2009(4): 14-15.
[13] 彭容秋. 有色金属提取冶金手册(锌镉铅铋)[M]. 北京: 冶金工业出版社, 1992.
PENG Rong-qiu. Hand-book on extractive metallurgy of No-ferrous metals (Zn, Cd, Pb, Bi)[M]. Beijing: Metallurgical Industry Press, 1992.
[14] 邹小平, 汪胜东, 蒋训雄, 蒋应平, 王海北, 刘三平. 铜镉渣提取镉绵工艺研究[J]. 有色金属: 冶炼部分, 2010(6): 2-5.
ZOU Xiao-ping, WANG Sheng-dong, JIANG Xun-xiong, JIANG Ying-ping, WANG Hai-bei, LIU San-ping. Process optimization research of extracting cadmium sponge from copper and cadmium residue[J]. Nonferrous Metals: Extractive Metallurgy, 2010(6): 2-5.
[15] 杜新玲, 张 欣, 马科友. 精镉的工业化生产[J]. 中国有色冶金, 2010(1): 25-29.
DU Xin-ling, ZHANG Xin, MA Ke-you. Industrialized production of pure cadmium[J]. China Nonferrous Metallurgy, 2010(1): 25-29.
[16] 虢振强. 真空蒸馏工艺生产镉的实践及改造[J]. 矿业工程, 2006, 26(3): 50-52.
GUO Zhen-qiang. Practices and transformation of production of cadmium by vacuum distillation process[J]. Mining and Metallurgical Engineering, 2006, 26(3): 50-52.
[17] HIRSCH H E, LIANG S C, WHITE A G. Chapter 2 preparation of high-purity cadmium, mercury, and tellurium[J]. Semiconductors and Semimetals, 1981, 18: 21-45.
[18] 邹学付, 陈卫华. 火法生产精镉工艺[J]. 技术与设备, 2007(9): 22-23.
ZOU Xue-fu, CHEN Wei-hua. Preparation of refined cadmium by pyrometallurgical process[J]. Technology and Equipment, 2007(9): 22-23.
[19] FUTAKI S, SHIRAISHI K, UEMURA M. Ultrafine refractory metal particles produced by hybrid plasma process[J]. Journal of the Japan Institute of Metals, 1992, 56(4): 4-64.
[20] VISWANATH S G, SAJIMOL G. Electrowinning of cadmium powder from glycerol and sulphuric acid—Study of morphology and nano-nature of the powder[J]. Metallurgija, 2010, 16(1): 25-38.
[21] JAVIER M G, JOSE M B. Removal of cadmium and production of cadmium powder using a continuous undivided electrochemical reactor with a rotating cylinder electrode[J]. Journal of Chemical Technology and Biotechnology, 2002, 77(4): 465-472.
[22] HATA Y, ONOUE M, SASAKI M, SENZAKI H, SUMIDA M, TANAKA J. Process for preparing metallic cadmium powder: US 5505761 A[P]. 1996-04-09.
[23] 李洪桂. 冶金原理[M]. 北京: 科学出版社, 2007: 329.
LI Hong-gui. Metallurgical theory[M]. Beijing: Science Press, 2007: 329.
(编辑 李艳红)
基金项目:广东省科技厅资助项目(2011B0508000033)
收稿日期:2013-07-09;修订日期:2013-09-12
通信作者:郑雅杰,教授,博士;电话:0731-88836285;E-mail: ZYJ@csu.edu.cn