硫酸盐溶液中二癸基次膦酸的钠皂萃取Co(II)动力学
来源期刊:中国有色金属学报(英文版)2013年第2期
论文作者:邢 鹏 王成彦 徐盛明 居中军
文章页码:517 - 523
关键词:二癸基次膦酸;钴;萃取动力学;萃取
Key words:di-decylphosphinic acid; cobalt; extraction kinetics; extraction
摘 要:用改进的Lewis槽研究了硫酸盐溶液中二癸基次膦酸(DDPA)的钠皂(Na-DDPA)萃取Co(II)动力学。分别考查了搅拌速度、温度、界面面积、钠皂浓度、钴离子浓度对萃取速率的影响。当搅拌速度在95~110 r/min范围时,扩散阻力对萃取速率的影响可以忽略。萃取速率随着温度和比界面面积的增大而增大。计算得活化能为32.75 kJ/mol。表明Na-DDPA萃取Co(II)为化学反应控制过程,Co(II)萃取的控制步骤为界面化学反应。萃取速率随着Na-DDPA和Co(II)浓度的增大而增大。得到了钴萃取动力学方程并讨论了萃取机理。
Abstract: Kinetics of Co(II) extraction from sulfate aqueous solution by the sodium salt of di-decylphosphinic acid (Na-DDPA) was studied using a modified Lewis cell to disclose the mechanism of extraction. Parameters affecting the extraction rate, such as stirring speed, temperature, interfacial area, Na-DDPA concentration and Co(II) concentration, were investigated, respectively. The effect of diffusion resistance on extraction rate was negligible when the stirring operation was conducted in a plateau region of 95-110 r/min. Extraction rate increased with the increase in the temperature or specific interfacial area. The activation energy E was calculated to be 32.75 kJ/mol. These suggested that rate controlling mechanism of Co(II) extraction by Na-DDPA was chemical reaction regime and the rate-determining step of Co(II) extraction was chemical reaction at the interface. The initial extraction rate also increased with the increase in the concentrations of Na-DDPA and Co(II). The extraction rate equation and mechanism of Co(II) extraction by Na-DDPA were proposed.
Trans. Nonferrous Met. Soc. China 23(2013) 517-523
Peng XING1, Cheng-yan WANG1, Sheng-ming XU2, Zhong-jun JU1
1. Department of Metallurgy, Beijing General Research Institute of Mining and Metallurgy, Beijing 100070, China;
2. Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China
Received 10 April 2012; accepted 23 May 2012
Abstract: Kinetics of Co(II) extraction from sulfate aqueous solution by the sodium salt of di-decylphosphinic acid (Na-DDPA) was studied using a modified Lewis cell to disclose the mechanism of extraction. Parameters affecting the extraction rate, such as stirring speed, temperature, interfacial area, Na-DDPA concentration and Co(II) concentration, were investigated, respectively. The effect of diffusion resistance on extraction rate was negligible when the stirring operation was conducted in a plateau region of 95-110 r/min. Extraction rate increased with the increase in the temperature or specific interfacial area. The activation energy E was calculated to be 32.75 kJ/mol. These suggested that rate controlling mechanism of Co(II) extraction by Na-DDPA was chemical reaction regime and the rate-determining step of Co(II) extraction was chemical reaction at the interface. The initial extraction rate also increased with the increase in the concentrations of Na-DDPA and Co(II). The extraction rate equation and mechanism of Co(II) extraction by Na-DDPA were proposed.
Key words: di-decylphosphinic acid; cobalt; extraction kinetics; extraction
1 Introduction
Researches on the kinetics of solvent extraction are very important to reveal the mechanism of the extraction process. In addition, it is interesting to find ways to improve the extraction rate via the investigation on kinetics. Detailed and exact information about extraction kinetics is necessary for the design of commercial solvent extraction process [1]. Up to now, there have been numerous literatures concerning the extraction equilibrium and selective extraction of Co(II) in the solvent extraction of Co(II) [2-9]. By contrast, only a few researchers studied the extraction kinetics of Co(II) by phosphoric/phosphonic acid extractants [10,11]. It is worth mentioning that these published literatures were not involved in the case of using sodium salt of extractant to extract Co(II). In the early work on mass transfer for the extraction of Co(II) from sulfate solution into Cyanex 272 [12], the extraction rate of Co(II) was found to increase with the increase of neutralization level of Cyanex 272 (the neutralization agent was NaOH). However, no attempt was made to determine the actual extraction mechanism.
Several techniques, such as highly stirred vessel, rotating diffusion cell, single drop and constant interfacial area stirred (Lewis) cell, are available for measuring the kinetic data of solvent extraction. Unfortunately, all these techniques have some drawbacks and need improvement [13]. In addition, extraction mechanism sometimes shows different results due to the differences in experimental technique and concentration of reactants [14-16]. These generally result in the difficulty in accurate measuring of kinetic data. For constant interfacial area stirred cell technique, aqueous phase and organic phase are separately stirred and the transfer occurs through the contact area [17]. One advantage of this technique is that the interfacial area is known and constant whereas the stirring speed can be varied in a wide range. The major limitation is that the interfacial turbulence is not well defined and varies with equipment. Nevertheless, reproducible data can be obtained by using well-designed equipment and careful operation [13].
Di-decylphosphinic acid (DDPA) is a recently-synthesised phosphinic acid based extractant. The extraction equilibrium and selective extraction of Co(II) by DDPA have been reported in our former published literatures [18,19]. DDPA was proved to be a promising extractant for Co/Ni separation in sulfate aqueous solution. In the solvent extraction process of Co(II), DDPA was generally converted to Na-DDPA before extracting Co(II). To do so can achieve high extraction efficiency of Co(II). The aim of this work was to disclose the mechanism and kinetics of Co(II) extraction from sulfate solution by Na-DDPA using constant interfacial area stirred cell technique.
2 Experimental
2.1 Chemicals and reagents
Cobalt sulfate solutions were prepared by dissolving cobalt(II) sulfate heptahydrate (analytical reagent grade) with deionized water. The pH of all Co(II) solutions was adjusted to 3.50 by adding dilute H2SO4. DDPA was produced in-house. Sulfonated kerosene was used as the diluent. The sodium salts of DDPA (Na-DDPA) were prepared by adding stoichiometric amount of 10 mol/L NaOH solution to the extractant in kerosene and stirring the phases to form a single phase.
2.2 Procedure
The extraction rate of Co(II) was measured using a constant interface stirred cell, which was a glass vessel. The cell consisted of two flat-blade paddles and two horizontal baffles. The interface was restricted to an annular gap between a central circular baffle and circumferential wall baffle. Three vertical baffles were fixed on the wall to prevent cavitation. Interfacial area was changed by using central circular baffles with different dimensions. The cell was placed in a thermostat bath to enable experiments to be carried out at various desired temperatures.
Preliminary experiments were first carried out to determine the optimum conditions for producing smooth interfaces at high stirring speeds. In each experiment, aqueous solution and the organic solution were preheated in advance at a desired temperature. An aqueous solution of 385 mL was first put into the cell. The top surface of aqueous phase was in the mid-point of the annulus. Subsequently, the same volume of organic solution was pumped through a tubule and slowly trickled along the wall as not to disturb the interface. The paddles were then driven by motors. The extraction was assumed to be initiated when the stirring was started. An organic phase of 5 mL was taken by using a glass syringe at certain time intervals and stripped by 15 mL sulfuric acid (H2SO4, 200 g/L). The metal concentration in aqueous strip solution was determined using atomic absorption spectrometry (AAS). The concentrations of Co(II) and Na-DDPA were fixed respectively at 0.006 and 0.078 mol/L, the interfacial area was fixed at 21.19 cm2 and the temperature was fixed at 299 K, unless otherwise stated.
3 Results and discussion
3.1 Effect of stirring speed on extraction rate
In general, the rate controlling mechanisms of the extraction include three basic regimes: diffusion regime, chemical reaction regime and mixed regime. The mass transfer controls the extraction rate in diffusion regime [20]. While in the chemical reaction regime, the kinetics of extraction is dependent on chemical reaction rather than mass transfer. For mixed regime, the mass transfer and chemical reaction control the extraction rate together.
As the case of liquid-solid reaction, diffusion of reactants to the interface and the diffusion of products from the interface also exist in the solvent extraction of metals from aqueous phase. Their rates depend on concentration gradient across a diffusion layer between the bulk solution and interface. It is known that changing stirring speed may cause the change of concentration gradient and then affect the diffusion rate. LEWIS [17] had also pointed out the importance of stirring speed to metal ion extraction kinetics. So in this work, the effect of stirring speed was firstly studied in the determination of extraction mechanism.
The stirring speed was varied in the range of 70-110 r/min to examine the effect on extraction rate. The plots of cobalt concentration in organic phase vs time at each stirring speed are shown in Fig. 1. The slope of tangent line to the curve at zero time was considered to be the value of initial extraction rate (r0). Figure 2 shows the plot of initial extraction rate vs stirring speed.
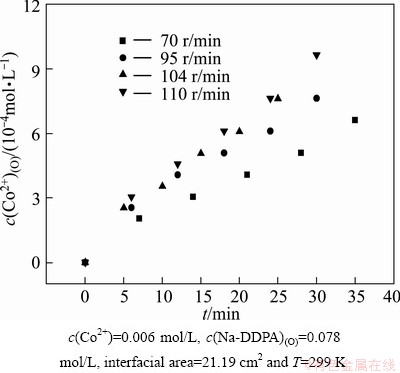
Fig. 1 Plots of c(Co2+)(O) vs time at different stirring speeds under conditions
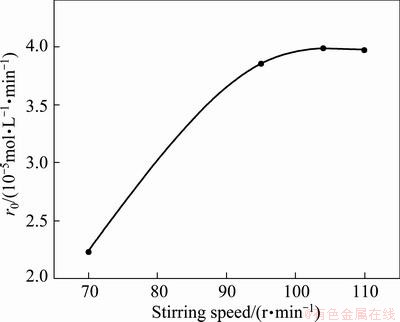
Fig. 2 Effect of stirring speed on extraction rate
It was observed that initial extraction rate increased rapidly with the increase of stirring speed up to 95 r/min. After that, the initial extraction rate gradually tended to stabilize with further increase of stirring speed. Figure 2 indicated a plateau region of 95-110 r/min. As stirring operation was conducted at the plateau region, the effect of diffusion resistance on extraction rate was minimized. Therefore, it could be assumed that the effect of mass transfer in this case had been eliminated and the extraction rate was mainly controlled by chemical reaction. The similar phenomenon was observed in the metal extraction by hydroxyoximes [15].
3.2 Effect of temperature on extraction rate
It is worth noting that temperature condition can alter the mechanism [21,22]. Generally, the extraction kinetics in high temperature region is different from that in low temperature region. Taking into account the common temperature range of the solvent extraction of cobalt, the temperature determined in this work was varied in a moderate range of 299-323 K. Experiments were carried out in the thermostat bath. As shown in Fig. 3, extraction rate increased with the increase in the temperature throughout the temperature range.
According to the Arrhenius equation, the relation- ship of r0 with temperature T can be expressed by
r0=Aexp[-E/(RT)] (1)
where E is the activation energy, R is the mole gas constant and A is the pre-exponential factor.
Taking the ln of Eq. (1) gives:
lnr0=–[E/(RT)]+ln A (2)
The lnr0 vs 1/T plot is shown in Fig. 4. The value of E was calculated from the slope of this line and determined to be 32.75 kJ/mol. The temperature effect on the rate of extraction controlled by chemical reaction is more pronounced than that controlled by the diffusion and the activation energy in the former case usually exceeds 21 kJ/mol [23,24]. The large value of E suggests that extraction of Co(II) is controlled by the chemical reaction occurring at the interface or in a region close to the interface.
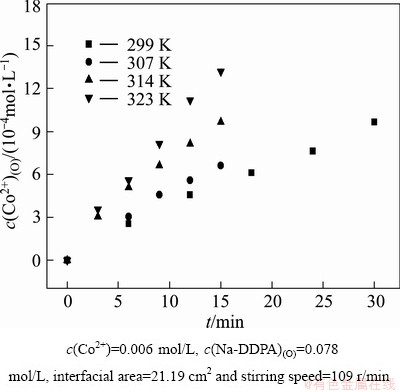
Fig. 3 Plots of c(Co2+)(O) vs time at different temperatures under conditions
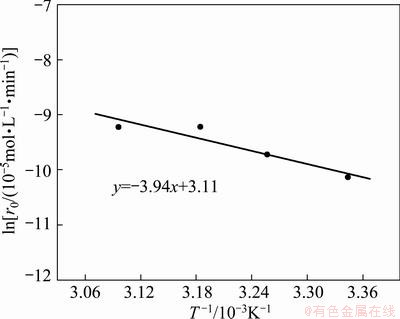
Fig. 4 Effect of temperature on extraction rate
Therefore, it could be concluded that the rate controlling mechanism of the Co(II) extraction by Na-DDPA was chemical reaction regime. The chemical reaction depended on the chemical factors such as bond energy and electronic structure of intermediate species [15].
3.3 Effect of interfacial area on extraction rate
The interfacial area is usually a parameter to differentiate chemical reaction taking place in the bulk phase or at the interface [20]. Experiments were carried out by changing interfacial area in the range of 8.64-25.12 cm2. The plots of c(Co2+)(O) vs time at different interfacial arens are shown in Fig. 5. As shown in Fig. 6, extraction rate increased with the increase of specific interfacial area (interfacial area/volume of the phase). The plot of r0 vs specific interfacial area yields a line through the origin of the axes. This indicates that extraction rate is dependent on the variation in interfacial area and the rate determining step of Co(II) extraction by Na-DDPA is chemical reaction at the interface.
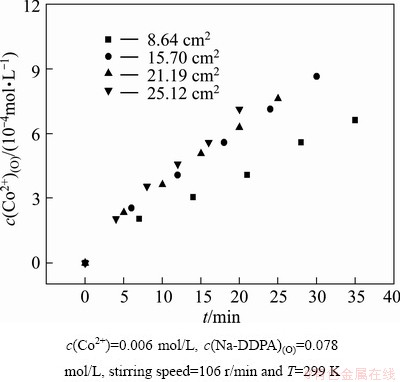
Fig. 5 Plots of c(Co2+)(O) vs time at different interfacial areas under conditions
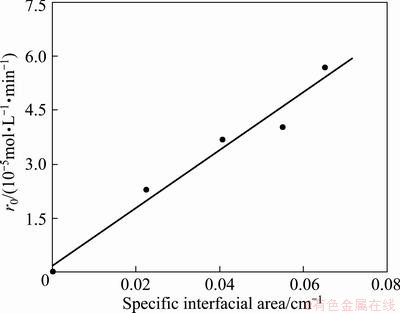
Fig. 6 Effect of interfacial area on extraction rate
3.4 Effect of Na-DDPA concentration on extraction rate
DDPA has a similar chemical structure with the Cyanex 272, so the following stoichiometric equation is used to describe the preparation of sodium salt of DDPA:
2NaOH+(HA)2(O)→2NaA(O)+2H2O (3)
where (o) indicates species in organic phase. At the initial contact time, concentration of the complex in the organic phase was low and the reaction was far away from equilibrium, so the reverse reaction was considered negligible [20]. The kinetics of the metal extraction depends upon the concentration of the reactant species at the reaction sites [25]. As the extraction is controlled by chemical reaction, the equation of extraction rate is given as
 (4)
(4)
where r is the extraction rate, k is the extraction rate constant, x and y are the reaction orders.
Taking the ln of Eq. (4) gives:
ln r=lnk+xln c(NaA)(O)+yln c(Co2+) (5)
To determine the reaction order x, the initial rate method was used in this work. A set of kinetic experiments were designed to change the initial concentration of Na-DDPA in the range of 0.078–0.195 mol/L while keeping the initial Co(II) concentration at 0.006 mol/L. The plots of c(Co2+)(O) vs time at different Na-DDPA concentrations are shown in Fig. 7. The extraction rate shown in Fig. 8 increased with increase of Na-DDPA concentration. The plot of lnr0 vs ln c(Na-DDPA)(O) yields a line with a slope of x, the order for Co(II). The slope of linear regression line, namely the value of x was determined to be 0.71.
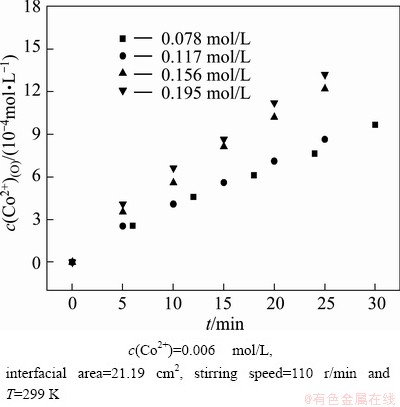
Fig. 7 Plots of c(Co2+)(O) vs time at different Na-DDPA concentrations under conditions
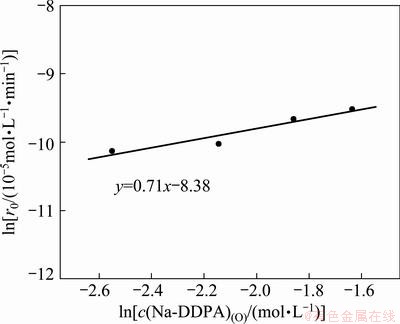
Fig. 8 Effect of Na-DDPA concentration on extraction rate
FU and GOLDING’s [12] research showed that the value of extraction rate of Co(II) in the case of Na-Cyanex 272 was much greater than that of Cyanex 272, and the extraction rate of Co(II) increased with the increase of neutralization level of Cyanex 272. A pertinent explanation was that the sodium salts of these extractants had a greater surface activity and the reverse micelles easy to react with metal ions were formed in the presence of the sodium salt [26]. Increasing the concentration of sodium salt of DDPA would create more opportunity for the extraction of cobalt ions and as a result the extraction rate increased. This also explains why metal extraction ratio generally increased with the increase of neutralization level of phosphorus based extractants.
3.5 Effect of cobalt concentration on extraction rate
Similarly, the concentration of cobalt was varied in the range of 0.006–0.013 mol/L while the concentration of Na-DDPA was kept at 0.117 mol/L to determine the reaction order y. The plots of c(Co2+)(O) vs time at different cobalt concentrations are shown in Fig. 9. The plot of ln r0 vs lnc(Co2+) is shown in Fig. 10. Extraction rate was found to increase with increasing Co(II) concentration. As the case of reaction order x, the reaction order y was determined to be 0.96. The average value of rate constant was calculated to be 2.75×10-2 mol-0.67·L0.67/min.
Under the conditions of interfacial area 21.19 cm2, temperature 299 K, Na-DDPA concentration 0.078–0.195 mol/L and Co(II) concentration 0.006–0.013 mol/L, the extraction rate equation of Co(II) can be represented by r=2.75×10-2c0.71(NaA)(O) c0.96(Co2+) .
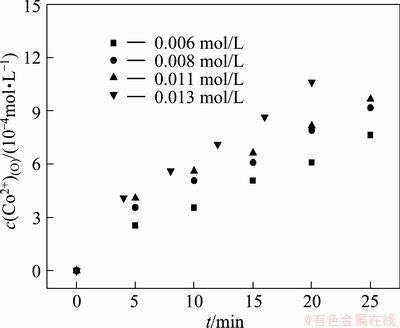
Fig. 9 Plots of c(Co2+)(O) vs time at different cobalt concentrations at c(Na-DDPA)(O)=0.117 mol/L, interfacial area=21.19 cm2, stirring speed=104 r/min and T=299 K
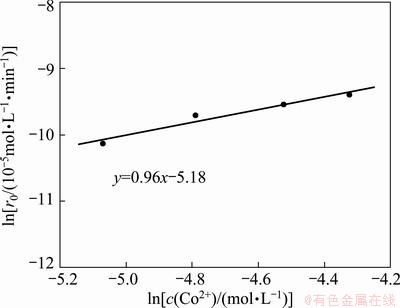
Fig. 10 Effect of cobalt concentration on extraction rate
3.6 Proposed mechanism of Co(II) extraction by Na-DDPA
According to the known research findings [13], the proposed mechanism of Co(II) extraction by phosphoric/phosphonic acid is as follows:
HA(O) HA(ad) (6)
HA(ad) (6)
HA(ad)
 ,
, 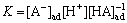 (7)
(7)
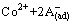
 CoA2(ad) (8)
CoA2(ad) (8)
CoA2(ad) CoA2(O) (9)
CoA2(O) (9)
where (ad) indicates species adsorbed at the liquid-liquid interface and K means the equilibrium constant.
The steps represented by Eqs. (7) and (8) are generally considered the slow steps when extraction rate is controlled by the chemical reaction, thus, the extraction rate is dependent on [H+]. However, in the case of sodium salt, as reported in extraction equilibrium of Co(II) [19], the distribution ratio of Co(II) almost unchanged in the [Na+] concentration range of 0.39-1.51 mol/L. In other words, [Na+] concentration in aqueous solution was found to have almost no effect on Co(II) extraction. Therefore, it means that the kinetic regimes of Co(II) extraction by their phosphinic acids and sodium salts are not the same. Based on the above kinetics results, the possible mechanism of Co(II) extraction by Na-DDPA is as follows:
NaA(O) NaA(ad) (10)
NaA(ad) (10)
NaA(ad)
 (11)
(11)
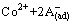
 CoA2(ad) (12)
CoA2(ad) (12)
CoA2(ad) CoA2(O) (13)
CoA2(O) (13)
The difference is that the step Eq. (12) here is the only slow step or rate-determining step.
4 Conclusions
Under conditions of the stirring speed range 95-110 r/min, temperature range 299-323 K, Na-DDPA concentration range 0.078-0.195 mol/L and Co(II) concentration range 0.006-0.013 mol/L, the rate controlling mechanism of the Co(II) extraction from sulfate solution by Na-DDPA was chemical reaction regime and the rate-determining step was chemical reaction at the interface. The effect of diffusion resistance on extraction rate was negligible when the stirring operation was conducted at the plateau region of 95-110 r/min. Extraction rate increased with the increase of temperature, specific interfacial area and concentrations of cobalt and Na-DDPA. The activation energy E was calculated to be 32.75 kJ/mol. The extraction rate equation can be represented by r=2.75×10-2c0.71(NaA)(O)c0.96(Co2+).
References
[1] INOUE K, GOYA M, TANIGUCHI M. Extraction equilibrium and extraction kinetics of nickel from aqueous ammonium nitrate solution with Versatic 10 in n-hexane [J]. Hydrometallurgy, 1984, 13(2): 155–167.
[2] DARVISHIA D, HAGHSHENASA D F, KESHAVARZ ALAMDARI E, SADRNEZHAADA S K, HALALI M. Synergistic effect of Cyanex 272 and Cyanex 302 on separation of cobalt and nickel by D2EHPA [J]. Hydrometallurgy, 2005, 77(3-4): 227-238.
[3] DEVI N B, NATHSARMA K C, CHAKRAVORTTY V. Separation and recovery of cobalt(II) and nickel(II) from sulphate solutions using sodium salts of D2EHPA, PC 88A and Cyanex 272 [J]. Hydrometallurgy, 1998, 49(1-2): 47-61.
[4] FU X, GOLDING J A. Solvent extraction of cobalt and nickel in bis(2,4,4-tri-methylpentyl) phosphinic acid, “Cyanex-272” [J]. Solvent Extraction and Ion Exchange, 1987, 5(2): 205-226.
[5] PARHI P K, PANIGRAHI S, SARANGI K, NATHSARMA K C. Separation of cobalt and nickel from ammoniacal sulphate solution using Cyanex 272 [J]. Separation and Purification Technology, 2008, 59(3): 310-317.
[6] PARK K H, REDDY B R, JUNG S H. Transfer of cobalt and nickel from sulphate solutions to spent electrolyte through solvent extraction and stripping [J]. Separation and Purification Technology, 2006, 51(3):265-271.
[7] REDDY B R, SARMA P V R B. Separation and recovery of cobalt and nickel from sulfate solutions of Indian Ocean nodules using Cyanex 272 [J]. Minerals and Metallurgical Processing, 2001, 18(3): 172-176.
[8] SARANGI K, REDDY B R, DAS R P. Extraction studies of cobalt (II) and nickel (II) from chloride solutions using Na-Cyanex 272. Separation of Co(II)/Ni(II) by the sodium salts of D2EHPA, PC88A and Cyanex 272 and their mixtures [J]. Hydrometallurgy, 1999, 52(3): 253-265.
[9] TAIT B K. Cobalt–nickel separation: The extraction of cobalt(II) and nickel(II) by Cyanex 301, Cyanex 302 and Cyanex 272 [J]. Hydrometallurgy, 1993, 32(3): 365-372.
[10] CHEN C Q, ZHU T. Kinetics of cobalt(II) extraction with EHEHPA in heptane from acetate system using an improved Lewis cell technique [J]. Solvent Extraction and Ion Exchange, 1994, 12(5): 1013-1032.
[11] DREISINGER D B, COOPER W C. Kinetics of cobalt and nickel extraction using HEHEHP [J]. Solvent Extraction and Ion Exchange, 1986, 4(2): 317-344.
[12] FU X, GOLDING J A. Equilibrium and mass transfer for the extraction of cobalt and nickel from sulfate solutions into bis(2,4,4-trimethylpentyl) phosphinic acid-“cyanex 272” [J]. Solvent Extraction and Ion Exchange, 1988, 6(5): 889-917.
[13] RYDBERG J, COX M, MUSIKAS C, CHOPPIN G R. Solvent extraction principles and practice [M]. Florida: CRC Press, 2004.
[14] BISWAS R K, HANIF M A, BARI M F. Kinetics of forward extraction of manganese(II) from acidic chloride medium by D2EHPA in kerosene using the single drop technique [J]. Hydrometallurgy, 1996, 42(3): 399-409.
[15] RICE N M, NEDVED M. On the mechanism of metal extraction by hydroxyoximes [J]. Hydrometallurgy, 1976, 2(4): 361-370.
[16] ZHU T. Solvent extraction in China [J]. Hydrometallurgy, 1991, 27(2): 231-245.
[17] LEWIS J B. The mechanism of mass transfer of solutes across liquid–liquid interfaces: Part I: the determination of individual transfer coefficients for binary systems [J]. Chemical Engineering Science, 1954, 3(6): 248-259.
[18] JU Zhong-jun, LI Lin-yan, XU Sheng-ming, WANG Cheng-yan, LIAO Fu-hui, LI Guo-bao. Synthesis and extraction performance of di-decylphosphinic acid [J]. Chinese Journal of Nonferrous Metals, 2010, 20(11): 2254-2259. (in Chinese)
[19] XING P, WANG C Y, JU Z J, LI D F, YIN F, CHEN Y Q, XU S M, YANG Y Q. Cobalt separation from nickel in sulfate aqueous solution by a new extractant: Di-decylphosphinic acid (DDPA) [J]. Hydrometallurgy, 2012, 113-114: 86-90.
[20] IRABIEN A, ORTIZ I, PEREZ DE ORTIZ E S. Kinetics of metal extraction: Model discrimination and parameter estimation [J]. Chem Eng Process, 1990, 27(1): 13-18.
[21] BISWAS R K, HABIB M A, ALI M R, HAQUE M Z. Kinetics of Mn2+ extraction in the acidic chloride D2EHPA–kerosene system using the constant interfacial area stirred cell technique [J]. Pak J Sci Ind Res, 1998, 41(3): 121-127.
[22] HUGHES M A, BISWAS R K. The kinetics of manganese(II) extraction in the acidic sulphate–D2EHPA–n-hexane system using the rotating diffusion cell technique [J]. Hydrometallurgy, 1993, 32(2): 209-221.
[23] SATO T, YOSHINO T, NAKAMURA T, KUDO T. The kinetics of aluminium(III) extraction from sulphuric acid solutions by di-(2-ethylhexyl)-phosphoric acid [J]. Journal of Inorganic and Nuclear Chemistry, 1978, 40(8): 1571-1574.
[24] EL-HEFNY N E. Kinetics and mechanism of extraction and stripping of neodymium using a Lewis cell [J]. Chemical Engineering and Processing, 2007, 46(7): 623-629.
[25] GAONKAR A G, NEUMAN R D. Interfacial activity, extraction selectivity, and reversed micellization in hydrometallurgical liquid/liquid extraction systems [J]. Journal of Colloid and Interface Science, 1987, 119(1): 251-261.
[26] MA Rong-jun. Solvent extraction in metallurgical processes [M]. Beijing: Metallurgical Industry Press, 2009: 496. (in Chinese).
邢 鹏1,王成彦1,徐盛明2,居中军1
1. 北京矿冶研究总院 冶金研究设计所,北京 100070;
2. 清华大学 核能与新能源技术研究院,北京 100084
摘 要:用改进的Lewis槽研究了硫酸盐溶液中二癸基次膦酸(DDPA)的钠皂(Na-DDPA)萃取Co(II)动力学。分别考查了搅拌速度、温度、界面面积、钠皂浓度、钴离子浓度对萃取速率的影响。当搅拌速度在95~110 r/min范围时,扩散阻力对萃取速率的影响可以忽略。萃取速率随着温度和比界面面积的增大而增大。计算得活化能为32.75 kJ/mol。表明Na-DDPA萃取Co(II)为化学反应控制过程,Co(II)萃取的控制步骤为界面化学反应。萃取速率随着Na-DDPA和Co(II)浓度的增大而增大。得到了钴萃取动力学方程并讨论了萃取机理。
关键词:二癸基次膦酸;钴;萃取动力学;萃取
(Edited by Hua YANG)
Foundation item: Projects (50734005, 51074096) supported by the National Natural Science Foundation of China; Project (2012AA06A110) supported by the Hi-tech Research and Development Program of China; Project (2012BAB07B01) supported by the National Key Technology R&D Program, China
Corresponding authors: Cheng-yan WANG; Tel: +86-10-63299551; E-mail: chywang@yeah.net; Sheng-ming XU; Tel: +86-10-62772055, E-mail: smxu@tsinghua.edu.cn
DOI: 10.1016/S1003-6326(13)62493-0

