文章编号:1004-0609(2007)11-1887-06
从锰银矿中一步法浸出锰和银的热力学基础与应用
张? 斌1,姜? 涛2,杨永斌2
(1. 湖南大学 材料科学与工程学院,长沙 410082;
2. 中南大学 资源加工与生物工程学院,长沙 410083)
摘? 要:研究在酸性介质中从锰银矿中同时浸出锰和银的热力学基础与技术条件。Mn-H2O系和Ag-H2O系电位( )—pH图研究结果表明:Mn2+和Ag+可以有一个稳定共存的区域,热力学条件为:25 ℃和101.325 kPa下,[Mn]= 1 mol/L,[Ag]=10?3 mol/L,pH<3.63,0.621 7<
)—pH图研究结果表明:Mn2+和Ag+可以有一个稳定共存的区域,热力学条件为:25 ℃和101.325 kPa下,[Mn]= 1 mol/L,[Ag]=10?3 mol/L,pH<3.63,0.621 7< <(1.229?0.118 2pH)和3.63<pH<4.635,0.621 7<
<(1.229?0.118 2pH)和3.63<pH<4.635,0.621 7< <(1.443 4?0.177 3pH)。过氧化氢的加入在一步法浸出过程中起着关键作用,在还原二氧化锰的同时还能同时使银氧化。加入高锰酸钾的作用是使矿石中未被二氧化锰包裹的银被氧化。室温下,浸出时间为2 h,高锰酸钾浓度为2 g/L,过氧化氢浓度为0.8 mol/L,硫酸浓度为0.9 mol/L,获得锰的浸出率为95.62%,银的浸出率为83.28%。
<(1.443 4?0.177 3pH)。过氧化氢的加入在一步法浸出过程中起着关键作用,在还原二氧化锰的同时还能同时使银氧化。加入高锰酸钾的作用是使矿石中未被二氧化锰包裹的银被氧化。室温下,浸出时间为2 h,高锰酸钾浓度为2 g/L,过氧化氢浓度为0.8 mol/L,硫酸浓度为0.9 mol/L,获得锰的浸出率为95.62%,银的浸出率为83.28%。
关键词:锰银矿;一步法浸出;电位( )—pH图;过氧化氢
)—pH图;过氧化氢
中图分类号:TF 843 ???? 文献标识码:A
Fundamentals and applications of simultaneous leaching of
manganese and silver from manganese-silver associated ore
ZHANG Bin1, JIANG Tao2, YANG Yong-bin2
(1. College of Materials Science and Engineering, Hunan University, Changsha 410082, China;
2. School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: Thermodynamics and technologies of simultaneous leaching of manganese and silver from manganese-silver associated ore in the sulfuric solution were investigated. Thermodynamics investigations of Mn-H2O system and Ag-H2O system show that there is a predominance area in potential ( )—pH diagram, where Mn2+ and Ag+ coexist in solution. The conditions are: 25 ℃ and 101.325 kPa, [Mn]=1 mol/L, [Ag]=10?3 mol/L, pH<3.63, 0.621 7<
)—pH diagram, where Mn2+ and Ag+ coexist in solution. The conditions are: 25 ℃ and 101.325 kPa, [Mn]=1 mol/L, [Ag]=10?3 mol/L, pH<3.63, 0.621 7< <(1.229?0.118 2pH) and 3.63<pH<4.635, 0.621 7<
<(1.229?0.118 2pH) and 3.63<pH<4.635, 0.621 7< <(1.443 4?0.177 3pH). The added hydrogen peroxide is critical to the simultaneous leaching, plays part of both the reducing agent of manganese dioxide and the oxidizing agent of native silver. The added potassium permanganate is for oxidizing the silver that is unwrapped by manganese dioxide in the ore. A recovery of 95.62% for Mn and 83.28% for Ag is attained at room temperature under the leaching conditions of 2 h leaching time, 2 g/L KMnO4, 0.8 mol/L H2O2 and 0.9 mol/L H2SO4.
<(1.443 4?0.177 3pH). The added hydrogen peroxide is critical to the simultaneous leaching, plays part of both the reducing agent of manganese dioxide and the oxidizing agent of native silver. The added potassium permanganate is for oxidizing the silver that is unwrapped by manganese dioxide in the ore. A recovery of 95.62% for Mn and 83.28% for Ag is attained at room temperature under the leaching conditions of 2 h leaching time, 2 g/L KMnO4, 0.8 mol/L H2O2 and 0.9 mol/L H2SO4.
Key words: manganese-silver associated ore; one-step leaching; potential ( )—pH diagram; hydrogen peroxide
)—pH diagram; hydrogen peroxide
????????????????????
目前,在国内相继发现了一批锰银矿床,其中锰矿物一般为软锰矿、硬锰矿等氧化矿物,锰主要以氧化锰形式存在,而银矿物一般有自然银、角银矿、银黝铜矿等,主要有4种赋存形式:一是以自然银形式存在于脉石中;二是以类质同象的形式赋存在锰矿晶格中;三是以胶体吸附状态存在于氧化锰矿石中;四是以高度分散状态被锰包裹,并且嵌布粒度较细,其中后3种形式比较普遍[1?3]。因此,难以通过机械选矿的方法得到高品位精矿,也难以通过单一氰化法获得好的浸出指标。目前已经公开报道的处理方法主要有以铅捕银法、氯化焙烧?氰化法、硫酸化焙烧?氰化法等[4?6]先火法后浸出的冶炼方法,以及二氧化硫?氰化法、硫酸亚铁?氰化法、有机物?氰化法等[7?10]两步浸出方法。这些方法共同缺点是:工艺流程复杂,生产周期长、劳动环境恶劣。为了缩短矿石处理的工艺流程和生产周期,优化劳动环境,在酸性条件下以过氧化氢溶液作为添加剂可以同时浸出锰和银,并且锰的浸出率较高,但银的浸出效果不理想[11?12]。本文作者从热力学角度出发,确定在酸性介质中添加过氧化氢和高锰酸钾同时浸出锰和银的可行性和主要技术条件,在此基础上在酸性条件下加入过氧化氢同时浸出锰和银,加入高锰酸钾以提高银的浸出率,进行条件优化实验。
1? 原料
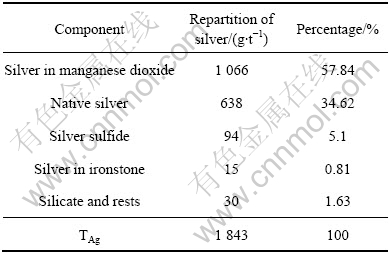
广西某矿区的多元素分析结果见表1。从表1可以看出,原矿中锰的含量只有12.20%,若单从锰的含量来看,属于贫锰矿,没有达到最低开采品位;银含量却很高,高达1 850 g/t,属于富银矿。此2种元素是该矿石的有用元素,因此银的回收应该是该矿石利用的重点,锰可以作为副产品加以回收。
表1 ?原矿多元素分析结果
Table 1? Chemical assay of raw materials (mass fraction, %)
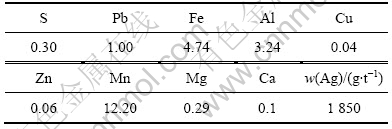
矿石中锰和银的物相组成见表2和表3。由表2可以看出原矿中锰的总含量达到11.90%,其中软锰矿含量最高,达到11.42%,占总含量的95.97%,是主要的存在形式;其次为硅酸锰,含量达到0.41%,占总含量的3.45%;最少的为碳酸锰,含量达0.07%,占总含量的0.58%。由表3可以看出,银的赋存形式比较多,主要是存在于氧化锰矿物中,达到1 066 g/t,占总量的57.84%,是以类质同象结构与氧化锰密切共生,或以胶体吸收态存在于氧化锰中,或以高度分散状态被氧化锰包裹,并且嵌布粒度较细;其次,尚有34.62%的银以自然银的形式存在于矿石之中,品位达638 g/t,是又一主要存在形式;另外还有少量铁矿中的银、硅酸盐及其它矿物中所含的银以及硫化银,含量非常少。
表2? 锰的物相组成
Table 2 ?Mineral phase constitutes of manganese

表3? 银的物相组成
Table 3 ?Mineral phase constitutes of silver
2? 一步法浸出锰和银的热力学基础
2.1? 一步法浸出锰和银的热力学研究
2.1.1 ?Mn-H2O系
25 ℃时,Mn-H2O系化学反应式和平衡方程式如下[13]:

根据以上数据,当p=101.325 kPa和[Mn]=1 mol/L时,计算后绘制出的Mn-H2O系 —pH图见图1。从图1可以看出,在pH值为0~12时,锰有MnO-4、MnO2、Mn2O3、Mn3O4、MnO、Mn2+、Mn共7个稳定存在的区域。当pH<8.904,溶液电位
—pH图见图1。从图1可以看出,在pH值为0~12时,锰有MnO-4、MnO2、Mn2O3、Mn3O4、MnO、Mn2+、Mn共7个稳定存在的区域。当pH<8.904,溶液电位 >?1.180时,Mn失去电子生成Mn2+进入溶液,当pH>8.904,
>?1.180时,Mn失去电子生成Mn2+进入溶液,当pH>8.904, >(?0.652 9?0.059 1pH),Mn可以氧化为MnO。当6.38<pH<8.904,
>(?0.652 9?0.059 1pH),Mn可以氧化为MnO。当6.38<pH<8.904, >(0.689 3?0.059 1 pH)时,Mn2+转变为Mn3O4,当3.63<pH<6.38,
>(0.689 3?0.059 1 pH)时,Mn2+转变为Mn3O4,当3.63<pH<6.38, >(1.443 4?? 0.177 3 pH)时,Mn2+转变为Mn2O3,当pH<3.63,?
>(1.443 4?? 0.177 3 pH)时,Mn2+转变为Mn2O3,当pH<3.63,?  >(1.229 0?0.118 2 pH)时,Mn2+转变为MnO2,当
>(1.229 0?0.118 2 pH)时,Mn2+转变为MnO2,当 >1.692 4?0.078 8 pH时,MnO2转变为Ag2O3。
>1.692 4?0.078 8 pH时,MnO2转变为Ag2O3。

图1? Mn-H2O系的 —pH图
—pH图
Fig.1  —pH diagram of Mn-H2O system (25 ℃, 101.325 kPa, [Mn]=1 mol/L)
—pH diagram of Mn-H2O system (25 ℃, 101.325 kPa, [Mn]=1 mol/L)
2.1.2? Ag-H2O系
25 ℃时,Ag-H2O系化学反应式和平衡方程式如下[14]:

根据以上数据,当p=101.325 kPa 和[Ag]=10?3 mol/L时,计算后绘制出的Ag-H2O系 —pH图见图2。从图2可以看出,在pH值为0~12时,银有Ag2O3、AgO、Ag+、Ag2O、Ag共5个稳定存在的区域。pH<6.33,
—pH图见图2。从图2可以看出,在pH值为0~12时,银有Ag2O3、AgO、Ag+、Ag2O、Ag共5个稳定存在的区域。pH<6.33, >0.6217时,Ag可以转变为Ag+进入溶液,当pH>6.33,
>0.6217时,Ag可以转变为Ag+进入溶液,当pH>6.33, >(1.173?0.059 1 pH)时,Ag可以氧化为Ag2O,6.435<pH<9.328,
>(1.173?0.059 1 pH)时,Ag可以氧化为Ag2O,6.435<pH<9.328, >(1.949 3?0.118 2 pH)时,Ag+转变为AgO,pH<6.435,
>(1.949 3?0.118 2 pH)时,Ag+转变为AgO,pH<6.435, >(1.758 5?0.088 6 pH)时,Ag+转变为Ag2O3。
>(1.758 5?0.088 6 pH)时,Ag+转变为Ag2O3。

图2? Ag-H2O系的 —pH图
—pH图
Fig.2?  —pH diagram of Ag-H2O system (25 ℃, 101.325 kPa, [Ag] =10?3 mol/L)
—pH diagram of Ag-H2O system (25 ℃, 101.325 kPa, [Ag] =10?3 mol/L)
2.1.3 ?H2O2-H2O系
25 ℃时,H2O2-H2O系化学反应式和平衡方程式如下[15]:

根据以上数据,当p=101.325 kPa和[H2O2]= 1 mol/L时,计算后绘制出的H2O2-H2O系 —pH图见图3。从图3可以看出,在pH值为0~12时,当
—pH图见图3。从图3可以看出,在pH值为0~12时,当 >(0.682?0.059 1 pH),H2O2被氧化为O2,当
>(0.682?0.059 1 pH),H2O2被氧化为O2,当 >(1.5?0.059 1 pH), H2O2被氧化为中间产物HO2,当
>(1.5?0.059 1 pH), H2O2被氧化为中间产物HO2,当  >(3.064?0.059 1),H2O2被氧化为中间产物O,当
>(3.064?0.059 1),H2O2被氧化为中间产物O,当  >(1.776?0.059 1 pH),H2O2可以得电子变成H2O。
>(1.776?0.059 1 pH),H2O2可以得电子变成H2O。

图3 ?H2O2-H2O系的 —pH图
—pH图
Fig.3?  —pH diagram of H2O2-H2O system (25 ℃, 101.325 kPa, [H2O2]=1 mol/L)
—pH diagram of H2O2-H2O system (25 ℃, 101.325 kPa, [H2O2]=1 mol/L)
为了确定锰和银的具体浸出条件,将图1、2和3的Mn-H2O系、Ag-H2O系和H2O2-H2O系的 —pH图叠加起来,得到图4所示的
—pH图叠加起来,得到图4所示的 —pH图。从图4可以看出,Mn2+和Ag+共同存在的区域为:在25 ℃下,pH<3.63,0.621 7<
—pH图。从图4可以看出,Mn2+和Ag+共同存在的区域为:在25 ℃下,pH<3.63,0.621 7< <(1.229?0.118 2 pH)和3.63<pH<4.635,0.621 7<
<(1.229?0.118 2 pH)和3.63<pH<4.635,0.621 7< <(1.443 4?0.177 3 pH) (图4中斜线部分)。
<(1.443 4?0.177 3 pH) (图4中斜线部分)。
2.2? 一步法浸出锰和银的化学反应
2.2.1? 锰浸出的化学反应
根据式(10)和(18),当 >1.692 4?0.078 8 pH时,MnO-4可以被H2O2还原成MnO2。总反应方程式为
>1.692 4?0.078 8 pH时,MnO-4可以被H2O2还原成MnO2。总反应方程式为

根据式(7)和(18)可知,当pH<3.63, >0.682?0.059 1 pH时,MnO2可以被H2O2还原成Mn2+进入到溶液中。总反应方程式为
>0.682?0.059 1 pH时,MnO2可以被H2O2还原成Mn2+进入到溶液中。总反应方程式为

根据式(9)和(18)可知,当pH>3.63, >0.682?0.059 1 pH时,MnO2可以被H2O2还原成Mn2O3。总反应方程式为
>0.682?0.059 1 pH时,MnO2可以被H2O2还原成Mn2O3。总反应方程式为

2.2.2? 银浸出的化学反应
从图4中可以看出,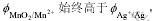 因此在热力学上MnO2可以氧化Ag。但对于锰银矿石来说,锰和银的存在形式虽然是MnO2和Ag,但Ag或被包裹或被吸附或以类质同象形式与二氧化锰密切共生,二者仍然能够长期稳定共存,并没有发生明显的氧化还原反应。这表明二者之间的化学反应从热力学角度来看是可以发生的,但由于二者在矿石中均以固体形式存在,反应速度太慢或反应界面条件的限制(如氧化膜的存在),导致二者之间长期共存。因此改善反应条件,如改善反应界面条件以提高有效扩散能力,降低反应活化能,提高有效碰撞次数,以加速化学反应的进行。
因此在热力学上MnO2可以氧化Ag。但对于锰银矿石来说,锰和银的存在形式虽然是MnO2和Ag,但Ag或被包裹或被吸附或以类质同象形式与二氧化锰密切共生,二者仍然能够长期稳定共存,并没有发生明显的氧化还原反应。这表明二者之间的化学反应从热力学角度来看是可以发生的,但由于二者在矿石中均以固体形式存在,反应速度太慢或反应界面条件的限制(如氧化膜的存在),导致二者之间长期共存。因此改善反应条件,如改善反应界面条件以提高有效扩散能力,降低反应活化能,提高有效碰撞次数,以加速化学反应的进行。

图4? Mn-H2O、Ag-H2O和H2O2-H2O系的 —pH图
—pH图
Fig.4? Overlapped —pH diagram of Mn-H2O, Ag-H2O and H2O2-H2O systems (Oblique line represents coexistence area of Mn2+ and Ag+)
—pH diagram of Mn-H2O, Ag-H2O and H2O2-H2O systems (Oblique line represents coexistence area of Mn2+ and Ag+)
从式(20)和(15)可以看出,酸性条件下过氧化氢可以直接氧化银并得到电子,其方程式为

但从式(18)和(15)可以看出,银离子又可以氧化过氧化氢变成单质银,其方程式为
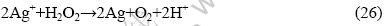
二者进行叠加,可得总反应方程式为

因此H2O2很难直接氧化Ag而生成银离子,单质银在H2O2分解中起催化剂的作用。
从式(19)可以看出,当 >(1.5?0.059 1 pH)时,H2O2可以被氧化生成中间产物HO2;而从式(15)可以看出,当有Ag存在时,HO2又可以氧化Ag而得到电子,使得Ag转变为Ag+进入溶液。因此,H2O2可以作为电子转移的载体改善化学反应条件,能够与MnO2发生反应失去电子,生成中间产物HO2(式(7)和(19)),而HO2再扩散到Ag的表面得到电子氧化Ag(式(19)和(15))。这样,MnO2和Ag均可以转变为可溶于水的Mn2+和Ag+,达到湿法冶金的目的。总的化学反应方程式为
>(1.5?0.059 1 pH)时,H2O2可以被氧化生成中间产物HO2;而从式(15)可以看出,当有Ag存在时,HO2又可以氧化Ag而得到电子,使得Ag转变为Ag+进入溶液。因此,H2O2可以作为电子转移的载体改善化学反应条件,能够与MnO2发生反应失去电子,生成中间产物HO2(式(7)和(19)),而HO2再扩散到Ag的表面得到电子氧化Ag(式(19)和(15))。这样,MnO2和Ag均可以转变为可溶于水的Mn2+和Ag+,达到湿法冶金的目的。总的化学反应方程式为
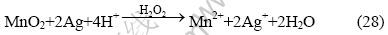
另外,在锰银矿石中,一般还有部分Ag没有被MnO2所包裹,因此即使H2O2进入溶液,仍然无法氧化这部分单质Ag。为了提高Ag的浸出率,有必要在溶液中加入一定量的氧化剂。而高锰酸钾是最好的一种强氧化剂,它能够溶解在水溶液中并扩散到Ag表面氧化Ag(式(10)和(15)),其还原产物也是Mn2+,不会在溶液中增加杂质。另外和MnO2一样,高锰酸钾可以氧化H2O2,生成中间产物HO2或O(式(10)、(19)和(21)),而中间产物HO2或O又可以扩散到Ag表面氧化Ag(式(19)、(21)和(15))。其总反应方程式为:

3? 一步法浸出锰和银的实验
? ?根据前面得到的热力学反应条件,确定反应体系为酸性介质,把KMnO4溶液加入锰银矿浆中,充分混合后加入H2O2溶液。条件实验结果如图5所示。为了节约化学药剂并同时获得满意的浸出率,确定最佳条件为:室温下浸出2 h,高锰酸钾浓度2 g/L,过氧化氢浓度0.8 mol/L,硫酸浓度0.9 mol/L。获得锰的浸出率为95.62%,银的浸出率为83.28%。
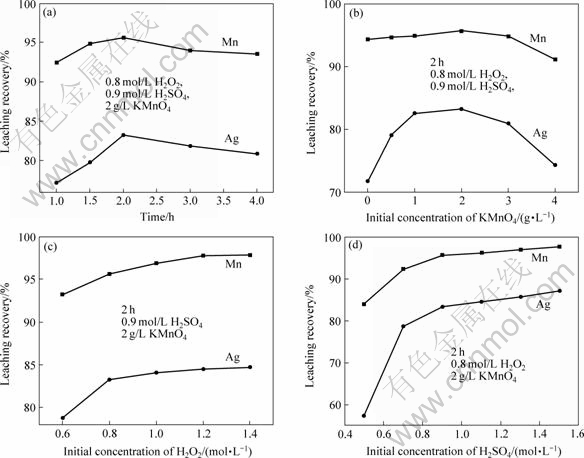
图 5? 一步法浸出锰和银的条件实验
Fig.5? Processing experiments of simultaneous leaching of manganese and silver: (a) Effect of time on leaching of Mn and Ag; (b) Effect of KMnO4 concentration on leaching of Mn and Ag; (c) Effect of H2O2 concentration on leaching of Mn and Ag; (d) Effect of H2SO4 concentration on leaching of Mn and Ag
4 ?结论
1) 通过控制溶液pH值和电位,可以使矿石中的MnO2和Ag同时浸出,以Mn2+和Ag+形式进入溶液中,实现一步浸出的目的。热力学条件是:25 ℃和101.325 kPa下,[Mn]= 1 mol/L,[Ag]= 10?3 mol/L,pH<3.63,0.6217< <(1.229?0.118 2 pH)和3.63<pH<4.635,0.621 7<
<(1.229?0.118 2 pH)和3.63<pH<4.635,0.621 7< <(1.443 4?0.177 3 pH)。H2O2的加入可以使二氧化锰的还原和银的氧化同时进行。加入高锰酸钾可以使矿石中未被二氧化锰包裹的银氧化。
<(1.443 4?0.177 3 pH)。H2O2的加入可以使二氧化锰的还原和银的氧化同时进行。加入高锰酸钾可以使矿石中未被二氧化锰包裹的银氧化。
2) 一步浸出法工艺步骤为:在酸性介质中把KMnO4溶液加入锰银矿浆中,充分混合后加入H2O2溶液。最佳工艺条件为:室温下浸出2 h,高锰酸钾浓度2 g/L,过氧化氢浓度0.8 mol/L,硫酸浓度0.9 mol/L。获得锰的浸出率为95.62%,银的浸出率为83.28%。
REFERENCES
[1] 杨洪英, 巩恩普, 杨 立. 广西某锰银矿银的赋存状态研究[J]. 贵金属, 2006, 27(1): 1?5.
YANG Hong-ying, GONG En-pu, YANG Li. Study on the occurrence state of silver in the manganese-silver concentrate in Guangxi[J]. Precious Metals, 2006, 27(1): 1?5.
[2] 韦丛中, 李维天, 陈晓玉. 广西凤凰山锰银氧化矿的工艺矿物学特征[J] .中国锰业, 2003, 21(3): 9?13.
WEI Cong-zhong, LI Wei-tian, CHEN Xiao-yu. The process mineralogy of Fenghuangshan manganese-bearing silver oxide ores Guangxi[J]. China’s Manganese Industry, 2003, 21(3): 9?13.
[3] 吕志成, 张培萍, 段国正, 郝立波, 董广华. 内蒙古额仁陶勒盖银矿床锰矿物的矿物学初步研究[J]. 矿物岩石, 2002, 22(3): 1?3.
LU Zhi-cheng, ZHANG Pei-ping, DUAN Guo-zheng, HAO Li-bo, DONG Guang-hua. Study on manganese minerals of E’rentaolegai silver deposit[J]. Mineral Petrol, 2002, 22(3): 1?3.
[4] 徐振芳. 从锰银矿中提取银的研究[J]. 中国锰业, 1992, 10(3): 33?37.
XU Zhen-fang. Process of abstracting silver from silver-manganese ore[J]. China’s Manganese Industry, 1992, 10(3): 33?37.
[5] 孙敬峰. 从某银锰矿中回收银[J]. 湿法冶金, 2002, 21(1): 25?27.
SUN Jing-feng. Recovery of silver from silver-manganite[J]. Hydrometallurgy of China, 2002, 21(1): 25?27.
[6] 吴文伟. 银锰精矿焙烧硫酸浸出提银新工艺[J]. 有色金属, 2004, 56(1): 48?50.
WU Wen-wei. Silver extraction from silver-manganese concentrate by roasting-leaching with sulfuric acid[J]. Nonferrous Metals, 2004, 56(1): 48?50.
[7] 张小云, 田学达, 刘小玲, 张东方. 银锰矿中银的回收新工艺[J]. 中国有色金属学报, 2006,16(5): 914?918.
ZHANG Xiao-yun, TIAN Xue-da, LIU Xiao-ling, ZHANG Dong-fang. A novel technique for silver recovery from silver-manganese ore[J]. The Chinese Journal of Nonferrous Metals, 2006, 16(5): 914?918.
[8] 袁明亮, 邱冠周, 王淀佐. 细粒嵌布锰银矿浸取中的超声强化作用[J]. 过程工程学报, 2002, 2(1): 21?25.
YUAN Ming-liang, QIU Guan-zhou, WANG Dian-zuo. Effect of ultrasonic wave on leaching manganese-containing silver ore with the binary ore leaching process[J]. The Chinese Journal of Process Engineering, 2002, 2(1): 21?25.
[9] 罗天盛. 化学浸出银锰矿的研究[J]. 中国锰业, 1997, 15(3): 26?29.
LUO Tian-sheng. A study on chemical leaching of silver-manganese ore[J]. China’s Manganese Industry, 1997, 15(3): 26?29.
[10] 余丽秀, 王秋霞, 李 琦, 孙亚光, 曹耀华. 有机还原剂处理银锰矿新工艺研究[J]. 矿产保护与利用, 2002, 2: 38?40.
YU Li-xiu, WANG Qiu-xia, LI Qi, SUN Ya-guang, CAO Yao-hua. Study on new technique of reduction processing Ag-Mn ore with organic reducing agent[J]. Conservation and Utilization of Mineral Resources, 2002, 2: 38?40.
[11] JIANG Tao, YANG Yong-bin, HUANG Zhu-cheng, ZHANG Bin, QIU Guan-zhou. Leaching kinetics of pyrolusite from manganese-silver ores in the presence of hydrogen peroxide[J]. Hydrometallurgy, 2004, 72(1/2): 129?138.
[12] JIANG Tao, YANG Yong-bin, ZHANG Bin, HUANG Zhu-cheng. Kinetics of silver leaching from manganese-silver associated ores in sulfuric acid solution in the presence of hydrogen peroxide[J]. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science, 2002, 33(6): 813?816.
[13] 梅光贵, 钟竹前. 湿法冶金新工艺[M]. 长沙: 中南工业大学出版社, 1994.
MEI Guang-gui, ZHONG Zhu-qian. New technology in hydrometallurgy[M]. Changsha: Central South University of Technology Press, 1994.
[14] 黎鼎鑫, 王永录. 贵金属提取与精炼[M]. 长沙: 中南大学出版社, 2003.
LI Ding-xin, WANG Yong-lu. Extraction and refinement of precious metals[M]. Changsha: Central South University of Technology Press, 2003.
[15] 梁英教, 车荫昌. 无机物热力学数据手册[M]. 沈阳: 东北大学出版社,1993.
LINAG Ying-jiao, CHE Yin-chang. Handbook of inorganic thermodynamics[M]. Shenyang: Northeast University Press, 1993.
??????????? ?????????????????????
基金项目:国家自然科学基金资助项目(59604002)
收稿日期:2007-03-22;修订日期:2007-07-02
通讯作者:张? 斌,博士;电话:0731-8906635;E-mail: zbparrick2002@hotmail.com
(编辑 彭超群)