电解铜箔表面结构及性能影响因素
黄友明,王平,黄永发
(江西省江铜-耶兹铜箔有限公司,江西 南昌,330096)
摘 要:对铜箔进行化学处理,考察阴极钛辊表面粗糙度及阴极钛辊的腐蚀对铜箔的性能及表面图像影响。研究结果表明:增加处理液中Cu2+浓度及提高电流密度,有利于表面粗糙度增加,抗剥离强度增大,蚀刻因子Ef降低。若同时降低浸泡复合液中Cu2+和Zn2+浓度,增加Sb2+浓度,则表面粗糙度及抗剥离强度降低,蚀刻因子增加;复合液中Sb2+浓度增加也能使表面粗糙度增加,蚀刻因子增加,但是,抗剥离强度基本没有变化。添加CuSO4后,阴极钛辊腐蚀速度下降,当CuSO4质量浓度达到20 g/L后,钛的耐腐蚀速度在0.050 mm/a以下;当钛辊表面粗糙度Rz降低时,电解铜箔表面相对平整,晶粒大小较均匀,排列较规则。
关键词:电解铜箔;化学处理;表面粗糙度;腐蚀
中图分类号:TG166 文献标志码:A 文章编号:1672-7207(2010)06-2162-05
Surface structure and performance of electrolytic copper foils
HUANG You-ming, WANG Ping, HUANG Yong-fa
(Jiangxi Copper-Yates Foil Corporation, Nanchang 330096, China)
Abstract: Effects of surface roughness and erosion of titanium cathode drum on performance of electrolytic copper foils and surface images were studied by chemical treatments. The results show that surface roughness and contradict debonding intensity increases and etch factorial (Ef) decreases with the increase of copper concentration and electric current density. When the concentration of copper and zinc of leached compound solution decreases, surface roughness and contradict debonding intensity decreases but etch factorial (Ef) increases. When the concentration of Sb2+ of leached compound solution increases, surface roughness and contradict debonding intensity increases but etch factorial (Ef) has litter change. The erosion rate of titanium cathode drum decreases when CuSO4 is added. When the mass concentration of CuSO4 is added up to 20 g/L, the erosion rate is less than 0.050 mm/a. Moreover, the surface of electrolytic copper foils is even and the size is well-proportioned and ranks regularly when surface roughness of titanium cathode drum (Rz) decreases.
Key words: electrolytic copper foils; chemical treatment; surface roughness; corrosion
高性能电解铜箔是一种缺陷少、晶粒细、表面粗化度低、强度和延展性高、厚度薄的铜箔。它经过适当的表面处理,在印刷电路板(PCB)制造中具有高蚀刻系数、低残铜率,可避免短路、适用于高频,可制成高密度细线化、薄型化、高可靠性PCB用的铜 箔[1-2]。近年来,我国形成了以广东东莞—深圳、江苏昆山—苏州地区为中心的两大电子工业生产基地。电子产业带动印刷电路板产业高速增长,促使铜箔消费量猛增[3-4]。据中国电子材料行业协会覆铜板分会 统计,2006年,我国铜箔市场需求量约14万t。目前,国内具有一定规模的电解铜箔生产厂家有15 家左右,总生产能力为8万t,出口3.9万t,进口10万t,尤其是高档电解铜箔几乎全部依赖进口,存在较大的生产缺口。另外, 今后几年受成本、市场及环境等各种因素的影响, 日、美、欧等国家和地区的电解铜箔生产也将逐步转向中国[3]。阴极辊是电解铜箔生产的关键设备,硫酸铜电解液中的铜离子在外电场的作用下电沉积到阴极辊表面,阴极辊做匀速圆周旋转,铜离子连续电沉积到阴极辊表面,沉积到一定厚度经剥离收成卷,连续生产出电解铜箔[4-6]。所以,有人将阴极辊称为电解铜箔生产的心脏[7]。电解铜箔是在阴极辊表面电沉积生成,是阴极辊表面晶体结构的延续。阴极辊表面形态决定了电解铜箔亮面的形态,是阴极辊表面状况的反映。阴极辊表面晶粒大小、几何形状、平整度、粗糙度直接影响到电解铜箔的亮面质量[8-11]。阴极辊表面晶粒粒径越小,则电解铜箔晶粒越细小,几何排列越均匀;反之,则越粗糙,铜箔表面极易氧化,铜箔亮面色泽不均,出现雪花斑,严重时甚至出现亮面铜粉、乌化现象。阴极辊表面状况不但直接影响电解铜箔的亮面品质,而且对毛面晶体结构有十分关键的影响[12-13]。金属表面的粗糙度不同导致在电解液中的电化学行为不同。表面越粗糙,晶格变大,实际面积变大,实际电流密度下降,阴极极化变小。严重时,造成部分的实际电极电位达不到铜的析出电位或偏低,导致电解铜箔晶粒大小不均、排列杂乱,甚至出现渗透孔[14-15]。如何提高电解铜箔质量,成为了我国电解铜箔业迅速发展的关键。在此,本文作者在生产经验的基础上,开展电解铜箔表面结构及性能影响因素的工艺研究。
1 实验
1.1 实验原料
实验所用钛材由宝鸡钛业股份有限公司提供,其化学成分(质量分数)如表1所示。
1.2 实验方法
首先,将电解铜箔用10%的硫酸溶液酸洗20 s,再水洗;然后,在常温下,在一定的电流密度及铜离子浓度下,用110 g/L硫酸浸泡12 s进行粗化处理;再在常温下用含重金属的复合液浸泡12 s固化后,用0.3% T-(2,3-环氧丙氧)丙基三甲氧基硅烷处理10 s,在90 ℃下烘干。 同时,考察工业纯钛在20% H2SO4及添加20 g/L CuSO4后,腐蚀速度的变化。
表1 钛材的化学成分
Table 1 Chemical compositions of TA1 plate %
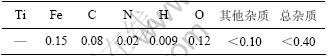
1.3 检测方法
镀层形貌采用KYKY2800电子显微镜观察。
将烘干的电解铜箔置于213 ℃的电烘箱中,于2 h后观察颜色变化,研究其高温抗氧化性能。
置于相对湿度90%,温度60 ℃的湿度实验箱中,于48 h后观察颜色变化,检测耐湿性能。
用玻璃环氧树脂板作为基板,在168 ℃下,用相当于FR-4 压力(3.8 MPa)加压90 min,然后剥离,测量抗剥离强度。
用覆铜板蚀刻制作线宽为50 μm和线间距为50 μm的精细线路,检测其腐蚀情况。
蚀刻因子为:

式中:H为铜箔厚度;WB为线路底部宽度;WT为线路顶部宽度。
2 结果与讨论
2.1 化学处理
在直流电的作用下, Cu2+被还原成Cu , 并在阴极辊上电化结晶形成生箔。为了获得较高的抗剥离强度,必须对生箔进行化学处理,其处理结果如表2所示,所得铜箔毛面结晶图像如图1所示。
表2 表面处理工艺条件与物性变化
Table 2 Technological conditions of surface treatment and physics quality of copper foils


图1 铜箔毛面结晶图像
Fig.1 Images of electrolytic copper foils
4种方法处理的铜箔高温抗氧化及耐湿性能检测后,均无颜色变化,表明高温抗氧化及耐湿性能较好。比较实验(b)和实验(a)发现:增加Cu2+质量浓度及提高电流密度,导致表面粗糙度增加,抗剥离强度增大,蚀刻因子降低。
比较实验(a)和实验(c)发现:同时降低浸泡复合液中Cu2+和Zn2+质量浓度,增加浸泡复合液中Sb2+质量浓度,则表面粗糙度及抗剥离强度降低,蚀刻因子增加。比较实验(c)和实验(d)发现:复合液中Sb2+质量浓度增加也能使表面粗糙度增加,蚀刻因子增加,但是,抗剥离强度基本没有变化。这说明浸泡复合液中Sb2+质量浓度的增加能导致蚀刻因子增加。
从图1也可以看出:实验(a)的铜箔粗糙度最大,实验(c)的铜箔粗糙度最小,说明粗化处理电流密度对其影响较大。这可能是在一定电流密度下,电结晶不同所致。
Bockris等[1]研究了CuSO4溶液中镀铜的交换电流密度i和过电位η的关系。根据塔菲尔方程:η=a±blg|i|,阳极过程和阴极过程具有不同的传递系数,即使η有微小变动也能导致电流密度i的改变很大。同样地,提高电流密度也能使过电位η增加,同时,电流密度的提高将使电化学极化及浓度极化增大,表面生成晶核数目增加,表面粗糙度Rz增加。而且在12 s粗化处理过程中,Cu2+质量浓度越高,生成晶核数目越多。
Rz越大,抗剥离强度越高,但它太高又容易造成蚀刻残铜[6]。适当降低溶液中Cu2+质量浓度和电流密度(大于极限电流),既可获得较低的Rz,又可使抗剥离强度基本不变。若Cu2+质量浓度和电流密度太低,则不能电解形成树枝状结构而是形成粉状,对生产不利,一般取Cu2+质量浓度为20~30 g/L、电流密度为25~35 A/dm2为最佳。
2.2 钛辊表面粗糙度对电解铜箔(亮面)的影响
图2和图3所示分别为钛辊表面粗糙度Rz不同时,得到的电解铜箔亮面及毛面结晶图像。从图2和图3可以看出:当钛辊表面粗糙度为0.4时,电解铜箔表面有明显凸起,亮面明显色泽不均,而且有聚集成团的趋势;而当钛辊表面粗糙度为0.2时,电解铜箔表面亮面相对平整、晶粒粒径均匀、排列规则。这说明金属表面的粗糙度不同,导致在电解液中的电化学行为不同。表面越粗糙,晶格越大,实际接触面积越大,而实际电流密度下降,阴极极化变小,严重时甚至会造成部分实际电极电位达不到铜的析出电位或偏低,导致电解铜箔晶粒粒径不均、排列杂乱,使电解铜箔表面明显凸起。而且高温抗氧化及耐湿性能检测后,粗糙度为0.2的试样均无颜色变化,而粗糙度为0.4的试样其抗氧化性较差。
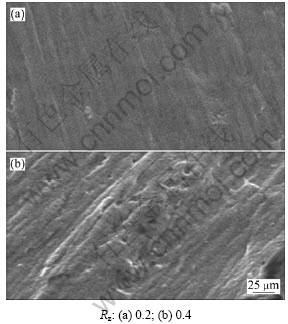
图2 不同钛辊表面粗糙度Rz下的电解铜箔亮面图像
Fig.2 Images of shine side surface of electrolytic copper foils with different surface roughness of titanium cathode drum

图3 不同钛辊表面粗糙度Rz下的电解铜箔毛面结晶毛面图像
Fig.3 Image of gross side surface of electrolytic copper foils with different surface roughness of titanium cathode drum
2.3 阴极辊的腐蚀
添加20 g/L CuSO4后,工业纯钛在20% H2SO4中的腐蚀速度如表3所示。钛在0℃时,可耐20%的H2SO4腐蚀,在室温只能耐5% H2SO4腐蚀[7]。从表3可见:工业纯钛在20%硫酸水溶液中腐蚀速度也相当高,达到61.8 mm/a;但是,当加入CuSO4溶液后,其腐蚀速度大大减低,而且随着加入CuSO4质量浓度的增加,其腐蚀速度下降,当其质量浓度达到20 g/L后,钛的耐腐蚀速度在0.050 mm/a以下。这是因为在电解铜箔生产中,阴极辊受到硫酸铜水溶液及电解过程的腐蚀,使阴极辊表面粗糙度增加,阴极过电位降低,铜离子在阴极表面电沉积结晶变粗,导致阴极析氢能力增大,对阴极辊表面形成氢腐蚀。钛在室温下能吸收大量的H2(470 mL/g),形成固溶体和固定组成的氢化物,使阴极辊腐蚀进一步加快,而且当溶液温度高,电流密度大,酸浓度高,铜离子浓度低,循环量不足时,阴极辊腐蚀加快。当加入铜后,补充了溶液中的铜离子,使铜离子优于氢而先析出,从而降低了阴极辊腐蚀速度。
表3 99 ℃时CuSO4质量浓度对工业纯钛腐蚀速度的影响
Table 3 Effect of concentration of CuSO4 on erosion rate of titanium cathode drum at 99 ℃

3 结论
(1) 增加Cu2+质量浓度及提高电流密度,表面粗糙度增加,抗剥离强度增大,蚀刻因子Ef降低。同时降低浸泡复合液中Cu2+和Zn2+质量浓度,增加Sb2+质量浓度,则表面粗糙度及抗剥离强度降低,蚀刻因子增加。复合液中Sb2+质量浓度增加,也能使表面粗糙度增加,蚀刻因子增加,但是,抗剥离强度基本没有变化。可见,浸泡复合液中Sb2+质量浓度的增加能导致蚀刻因子增加。
(2) 当钛辊表面粗糙度为0.4时,电解铜箔表面有明显凸起,亮面明显色泽不均,而且有聚集成团的趋势;而当钛辊表面粗糙度为0.2时,电解铜箔表面相对平整,晶粒粒径均匀,排列规则。
(3) 随着加入CuSO4质量浓度的增加,其腐蚀速度下降,当其质量浓度达到20 g/L后,钛的耐腐蚀速度在0.050 mm/a以下。
参考文献:
[1] Bockris J O M, Reddy A K N, Gamboa-Aldeco M. Modern electrochemistry seconded[M]. New York: Kluwer Academic, 2000: 10-12, 66.
[2] Schlesinger M, Paunovac M. Modern electroplating[M]. New York: Wiley, 2000: 47-52.
[3] 陈平华. 电解铜箔市场研究报告[J]. 世界有色金属, 2005(5): 19-27
CHEN Ping-hua. Market research of electrolytic copper foils[J]. World Nonferrous Metals, 2005(5): 19-27.
[4] 韩明臣. 钛合金的焊缝组织[J]. 稀有金属快报, 2003, 22(12): 23-24.
HAN Ming-chen. Structure of welding line of titanium alloy[J]. Rare Metals Letters, 2003, 22(12): 23-24.
[5] 任连保, 吴丕杰. 电解铜箔用钛焊接阴极辊焊缝均晶化处理工艺研究[J]. 稀有金属快报, 2007, 26(9): 39-41.
REN Lian-bao, WU Bi-jie. A study on grain refining treatment for welding seam of titanium cathode drum for electrolytic copper foil[J]. Rare Metals Letters, 2007, 26(9): 39-41.
[6] 黄洁. 铜箔的生产技术及发展趋向[J]. 铜业工程, 2003(2): 83-86.
HUANG Jie. Technology of copper foil manufacturing and its development tendency[J]. Copper Engineering, 2003(2): 83-86.
[7] Changa H K, Choeb B H, Lee J K. Influence of titanium oxide films on copper nucleation during electrodeposition[J]. Materials Science and Engineering A, 2005, 409(1/2): 317-328.
[8] Seol K W, Choe B H, Lee Y K, et al. Effect of metals on the mechanical properties of copper[J]. Mater Sci Forum, 2003, 426/432: 3715.
[9] Choe B H, Seol K W, Jeannette J A, et al. Copper electrochemistry and electrodeposition[C]//LiMAT-2003. Pohang: Postech, 2004: 237.
[10] Grosgogeat B, Reclaru L, Lissac M, et al. Measurement and evaluation of galvanic corrosion between titanium/Ti6Al4V implants and dental alloys by electrochemical techniques and auger spectrometry[J]. Biomaterials, 1999(20): 933-938.
[11] Rohly K, Istephanous N, Belu A, et al. Oxide films on metallic biomaterials: Myths, facts and opportunities[J]. Mater Sci Forum, 2003, 30(17): 426-432.
[12] Madhavan V, Chandrasekar S, Farris T N. Machining as a wedge indentation[J]. Journal of Applied Mechanics, 2000, 67(1): 128-139.
[13] Stein D F, Low Jr J R. Effects of orientation and carbon on the mechanical properties of iron single crystals[J]. Acta Metallurgica, 1966, 14(10): 1183-1194.
[14] Rohrer G S. Structure and bonding in crystalline materials[M]. Cambridge: The Press Syndicate of the University of Cambridge, 2001: 22-28.
[15] Tanaka Y. Oxide films on metallic biomaterials and alloy[EB/OL]. [2008-12-25]. http://dent.sympo.nagasaki- u.ac.jp/baylor/tanaka/ presentation.html.
(编辑 赵俊)
收稿日期:2010-03-06;修回日期:2010-06-08
基金项目:国家科技支撑计划项目(2007BAB22B002)
通信作者:王平(1970-),男,江西新余人,高级工程师,从事金属材料深加工研究;电话:0791-8198536;E-mail: wpoe_12345@163.com