高镁锂比盐湖卤水镁锂分离工艺
徐 徽,许 良,陈白珍,石西昌,杨 鑫
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘 要:以高镁锂比盐湖卤水为原料,进行镁锂分离的工艺研究。分别用氨和碳酸氢铵进行二段沉镁,卤水中98%的Mg2+以氢氧化镁和碱式碳酸镁形式沉淀分离出去,溶液经浓缩结晶析出氯化铵后, 再用氢氧化钠进行深度除镁,很好地实现镁锂的分离。研究NaOH的加料方式、浓度、加料速度、终点pH值、反应温度及反应时间对除镁效果的影响。实验结果表明:在NaOH溶液浓度为10 mol/L,加料速度为0.3~0.5 mL/min,pH =12,温度为 25 ℃,反应时间为20~30 min的条件下,母液中Mg2+可以除尽,为后续工序制备碳酸锂创造了有利的条件。
关键词:盐湖卤水;氢氧化镁;溶解度;浓缩
中图分类号:TQ123.4 文献标识码:A 文章编号:1672-7207(2009)01-0036-05
Separating technique for magnesium and lithium from
high Mg/Li ratio salt lake brine
XU Hui, XU Liang, CHEN Bai-zhen, SHI Xi-chang, YANG Xin
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Using salt lake brine with high magnesium/lithium ratio as raw material, the process of separating lithium from magnesium in the brine was studied. The technological parameters of the process were obtained. Two precipitation steps were studied in the process using ammonia and ammonium bicarbonate as the precipitator. About 98% of Mg2+ in the brine is precipitated as magnesium hydroxide and the light magnesium carbonate is precipitated respectively. NH4Cl is obtained while the solution is concentrated. And then, the magnesium ion remained in the solution is further extracted out by adding NaOH. The effect of some parameters of the process were studied and the effects of adding method of reagents, concentration of NaOH solution, adding rate of NaOH solution, terminal pH, reactive temperature and reactive time on magnesium extracting rate were investigated. The results show that under the conditions of 10 mol/L NaOH solution, adding rate 0.3-0.5 mL/min, pH 12, temperature 25 ℃, reactive time 20-30 min, Mg2+ is extracted from the salt lake brine completely. The treated solution can be used for preparation for high purity lithium carbonate. ⊙
Key words: saline lake brine; magnesium hydroxide; solubility; concentrate
自然界中锂资源主要赋存于花岗伟晶岩型矿床、盐湖卤水、海水及地热水中。据统计,盐湖卤水中锂资源储量约占锂资源总量的70%~80%,随着近年来锂矿石资源的日益枯竭,盐湖卤水提锂已成为锂盐生产和研究的主攻方向[1-2]。我国盐湖含锂卤水资源有2个显著的特点,一是锂含量高,卤水中锂质量浓度高达2.2~3.1 g/L;二是镁锂比高,比国外高数十倍乃至百倍,给锂资源的开发带来了一定的难度,因而,解决锂资源的高效分离提取技术,是开发我国盐湖锂资源的关键问题[3-4]。目前,盐湖卤水除镁提锂的方法主要有沉淀法、煅烧浸取法、离子交换法、溶剂萃取法等[5]。沉淀法和煅烧浸取法存在工艺流程复杂和耗能高等缺点,而离子交换法和溶剂萃取法由于缺乏高效的离子交换剂和萃取剂难以实现工业化。本文作者采用氨法二段沉镁和氢氧化钠深度除镁,以期实现镁锂分离。
1 实 验
1.1 实验原料
实验中所用的主要原料为青海某盐湖经自然蒸发浓缩、并经硫酸法制硼酸后的脱硼卤水,主组分为氯化镁和氯化锂,Mg2+和Li+质量浓度分别为110和2.5 g/L,主要杂质为钾、钠等的氯化物及少量硫酸盐[6]。实验中所用其他原料包括氨水、碳酸氢铵、氢氧化钠等,均为化学纯试剂。
1.2 实验原理和方法
1.2.1 一段沉镁
将卤水与氨水进行反应,通过控制反应温度、反应时间及加料速度等条件,使生成的氢氧化镁平均粒径达50 μm以上,其过滤洗涤性能好,滤饼含水率低(12%以下)。经分离氢氧化镁后得到的母液中Mg2+和Li+质量浓度分别为12和2.5 g/L,氯化铵质量浓度约350 g/L,实现了镁、锂的初步分离[7-8]。
主要反应式为:
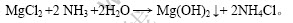
1.2.2 二段沉镁
一段沉镁后的母液冷却到室温后,会析出大量的氯化铵结晶,经过滤分离氯化铵后,加入2倍镁离子总量(摩尔数)的碳酸氢铵,在室温条件下进行反应,生成碳酸镁复盐(MgCO3·(NH4)2CO3·4H2O)沉淀,分离碳酸镁复盐后的母液中Mg2+和Li+质量浓度均为2.5 g/L左右,实现了镁、锂的二次分离[9-10]。
主要反应式为:
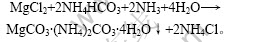
1.2.3 母液浓缩结晶分离氯化铵
经2次除镁后的母液主要成分为氯化铵、氯化锂和氯化镁,根据其在水中溶解度的差别,通过不断浓缩、结晶可分离出大部分氯化铵,最终使锂离子质量浓度达到25~30 g/L,镁离子质量浓度为20 g/L左右。
1.2.4 深度除镁
卤水经二段沉镁及浓缩结晶后,所得溶液采用氢氧化钠溶液进一步进行深度除镁,以实现镁、锂的彻底分离[11-12]。
主要反应式为:
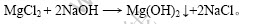
2 分析与讨论
2.1 NH4Cl,LiCl和MgCl2 的溶解度
在温度为20℃,Mg2+质量浓度分别为0,15,20和30 g/L条件下,进行NH4Cl和LiCl的溶解度试验,结果如图1所示。从图1可以看出,当体系中Mg2+质量浓度固定不变时,NH4Cl的溶解度随LiCl浓度的增加而减小;当体系中Li+固定不变时,NH4Cl的溶解度随Mg2+质量浓度的增加而减小。Mg2+质量浓度的增加和LiCl质量浓度的增加都可以大大降低NH4Cl在LiCl-MgCl2-H2O体系中的溶解度。
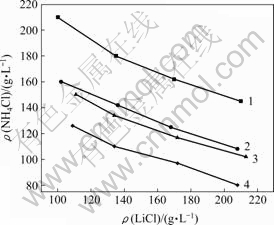
ρ(Mg2+)/(g?L-1): 1—0; 2—15; 3—20; 4—30
图1 NH4Cl在LiCl-MgCl2-H2O体系中的溶解度
Fig.1 Solubilities of NH4Cl in LiCl-MgCl2-H2O
2.2 沉镁母液蒸发浓缩实验
以上面的NH4Cl,LiCl和MgCl2 溶解度关系试验结果为指导,对卤水经一段、二段沉镁后的母液进行蒸发浓缩试验,对浓缩液进行取样分析,主要数据见表1。
表1 沉镁母液浓缩过程各物质的变化
Table 1 Change of substances in process of mother liquid concentration
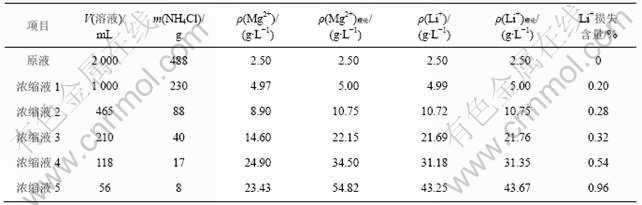
从表1可以看出,当Mg2+质量浓度接近10 g/L时,浓缩液中Mg2+质量浓度小于理论浓度,说明在蒸发过程中并不完全依据图1的规律进行,此时开始析出氨光卤石。当Li+质量浓度达30 g/L后,虽然NH4Cl可以继续析出,但Mg2+浓度变化不大,而且锂的损失逐渐加大,因此,蒸发浓缩时,以达此限度为宜。浓缩最后所得溶液Mg2+质量浓度约为23 g/L,Li+质量浓度约为30 g/L。
2.3 氢氧化钠法深度除镁实验
对Mg-H2O系电位—pH图(图2)进行分析,图中分为沉淀区、浸出区和净化区3个区域。对净化液除镁而言,当浸出液中的pH值调整到Mg(OH)2净化区时,镁离子将以Mg(OH)2形态从浸出液中析出;当 pH>10.47时,浸出液中的c(Mg2+)<10-4 mol/L,Mg2+在浸出液中含量很小。实践中,一般控制溶液的pH≥12。因此,本研究选用氢氧化钠进行深度除镁[13-15]。
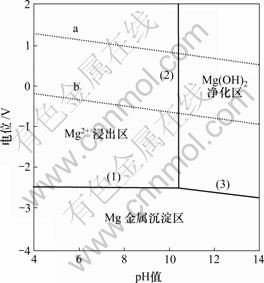
图2 Mg-H2O系电位—pH图
Fig.2 Potential—pH diagram of Mg-H2O
2.3.1 氢氧化钠加料方式的选择
考察了直接加入氢氧化钠固体和氢氧化钠溶液2种方式。固定实验条件为:1 L反应溶液,ρ(Mg2+)=23 g/L,ρ(Li+)=30 g/L,氯化铵饱和,快速搅拌,终点pH值控制在12,反应温度为25 ℃,反应时间为30 min。实验证明,当直接加入氢氧化钠固体时,由于反应时易形成氢氧化镁胶体,导致反应不完全,消耗大量的氢氧化钠。而氢氧化钠以溶液方式加入时,只要控制一定的加料速度,可以得到沉降及过滤性能较好的氢氧化镁沉淀。因此,选择溶液加料方式。
2.3.2 氢氧化钠溶液浓度的影响
固定实验条件为:1 L反应溶液,ρ(Mg2+)=23 g/L,ρ(Li+)=30 g/L,氯化铵饱和,终点pH值=12,反应温度为25 ℃,反应时间为30 min,进行单因素实验,结果见图3。可见,NaOH浓度对除镁效果基本没有影响,但当NaOH浓度较低时,会增大反应溶液体积并增加碱耗,不利于后续提锂;而当浓度NaOH浓度大于10 mol/L时,浓度越大,反应越容易产生胶体,将不利于过滤。由图3可得,选择NaOH浓度为10 mol/L较合适。
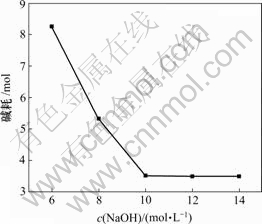
图3 碱耗与NaOH浓度的关系
Fig.3 Relationship between alkali consumption and concentration of NaOH
2.3.3 加料速度的影响
固定实验条件为:1 L反应溶液,ρ(Mg2+)=23 g/L,ρ(Li+)=20 g/L,氯化铵饱和,快速搅拌,终点pH值=12,反应温度为25 ℃,反应时间为30 min。实验结果见图4。可见,滴加速度在3~5 mL/min是合适的,在这个速度范围内可以保证反应后溶液有较好的流动性,有利于氢氧化镁的过滤。
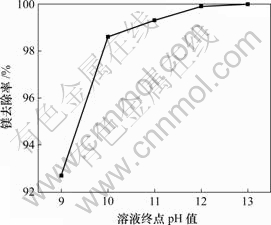
图4 镁去除率与溶液终点pH值关系
Fig.4 Relationship between removal rate of Mg and terminal pH of solution
2.3.4 终点pH值的影响
由前面的实验可知,溶液最终的pH值是除镁的一个关键因素。固定实验条件为:1 L反应溶液,ρ(Mg2+)=23 g/L,ρ(Li+)=30 g/L,氯化铵饱和,快速搅拌,反应温度为25 ℃,反应时间为30 min。从图4可以看出,要想彻底将镁去除,溶液终点的pH值需控制在12以上。
2.3.5 反应温度的影响
固定实验条件为:1 L反应溶液,ρ(Mg2+)=23 g/L,ρ(Li+)=30 g/L,氯化铵饱和;NaOH浓度为10 mol/L,反应时间为30 min。碱耗—反应温度曲线如图5所示。
由图5可知,当温度升高时,在镁离子完全去除时,溶液的终点pH值虽然可以降低,但是,碱的消耗却大大增加。这是因为温度升高时,有利于溶液中的铵根转化成氨气,一部分溢出,一部分与水结合成氨水,然后,在溶液体系中形成NH4Cl-NH3HO缓冲体系,从而耗掉大量的碱。因而,选择在常温下进行除镁比较合适。
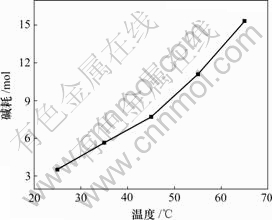
图5 碱耗与反应温度的关系
Fig.5 Relationship between alkali consumption and reaction temperature
2.3.6 反应时间的影响
这里讨论的反应时间是指加完料后的反应时间。为了观察反应时间对镁去除率的影响,固定实验条件为:1 L反应溶液,ρ(Mg2+)=23 g/L,ρ(Li+)=30 g/L,氯化铵饱和,快速搅拌,终点pH值为12,反应温度为25 ℃,反应时间对镁去除率的影响如图6所示。
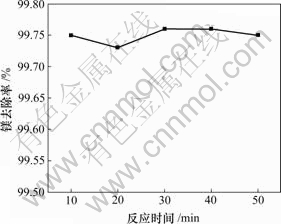
图6 镁去除率与反应时间的关系
Fig.6 Relationship between removal rate of Mg and reaction temperature
上述结果表明,在反应溶液pH值达到12以后,镁去除率已经很高,因此,延长反应时间并不能提高镁的去除率。故采用的时间以10~20 min为宜。
3 结 论
a. 盐湖脱硼卤水先通过二段沉镁,98%的Mg2+以氢氧化镁和碱式碳酸镁形式沉淀分离出去,再通过不断浓缩结晶以除去大部分氯化铵,同时富集Li+质量浓度达到30 g/L。
b. 用氢氧化钠溶液进行深度除镁,1 L反应溶液,在NaOH溶液浓度为10 mol/L,加料速度为3~5 mL/min, pH =12,温度为25 ℃,反应时间10~20 min的工艺条件下,溶液中Mg2+可以除尽,为后续工序制备碳酸锂创造了有利条件。
参考文献:
[1] 刘元会, 邓天龙. 国内外从盐湖卤水中提锂工艺技术研究进展[J]. 世界科技研究与发展, 2006(5): 69-75.
LIU Yuan-hui, DENG Tian-long. Progresses on the process and technique of lithium recovery from salt lake brines around the world[J]. World SCI-Tech R&D, 2006(5): 69-75.
[2] 高世杨. 青海盐湖锂盐开发与环境[J]. 盐湖研究, 2000, 8(1): 17-23.
GAO Shi-yang. Exploring and envierment of lithium of Qinghai salt lake[J]. Journal of Salt Lake Research, 2000, 8(1): 17-23.
[3] 汪镜亮. 卤水锂资源提锂现状[J]. 化工矿物与加工, 1999(12): 1-5.
WANG Jing-liang. The present status of lithium extraction from Li-bearing brines[J]. Industrial Minerals and Processing, 1999(12): 1-5.
[4] 于明臻. 我国锂盐工业的现状及技术进展[J]. 无机盐工业, 1999, 31(1): 21-24.
YU Ming-zhen. Present condition and technology progress of lithium industry in China[J]. Inorganic Chemical Industry, 1999, 31(1): 21-24.
[5] 孙锡良, 陈白珍, 徐 徽, 等. 从盐湖卤水中萃取锂[J]. 中南大学学报: 自然科学版, 2007, 38(2): 262-266.
SUN Xi-liang, CHEN Bai-zhen, XU Hui, et al. Extraction of lithium from bittern[J]. Journal of Central South University: Science and Technology, 2007, 38(2): 262-266.
[6] 汪贵元. 察尔汗盐湖镁资源及开发利用[J]. 无机盐工业, 2002, 34(3): 37-38.
WANG Gui-yuan. Magnesium resource in Chaerhan Salt Lake and its exploitation and utilization[J]. Inorganic Chemicals Industry, 2002, 34(3): 37-38.
[7] 徐日瑶. 镁冶金学[M]. 北京: 冶金工业出版社, 1981.
XU Ri-yao. Mg metallurgy[M]. Beijing: Metallurgical Industry Press, 1981.
[8] 徐日瑶. 有色金属提取冶金手册—镁[M]. 北京: 冶金工业出版社, 1992.
XU Ri-yao. A handbook for extractive metallurgy of nonferrous metals-Mg[M]. Beijing: Metallurgical Industry Press, 1992.
[9] 胡庆福. 我国轻质碳酸镁、轻质氧化镁生产现状及其发展[J]. 化工科技市场, 2001(6): 19-22.
HU Qing-fu. Production actualities and developments of light magnesium carbonate and light magnesium oxide[J]. Market of Science and Technology of Engineering Chemistry, 2001(6): 19-22.
[10] 徐日瑶, 刘宏专. 盐湖水氯镁石制取金属镁及高纯镁砂的生产技术[J]. 盐湖研究, 2003, 11(2): 46-50.
XU Ri-yao, LIU Hong-zhuan. Technology for the production of magnesium ang high-purity magnesite clinker using bischofite from Salt Lake[J]. Journal of Saltlake Research, 2003, 11(2): 46-50.
[11] 徐 徽, 蔡 勇, 陈白珍, 等. 用低品位菱镁矿制取高纯镁砂[J]. 中南大学学报: 自然科学版, 2006, 37(4): 698-702.
XU Hui, CAI Yong, CHEN Bai-zhen, et al. Preparation of high purity magnesia from low-grade magnesite[J]. Journal of Central South University: Science and Technology, 2006, 37(4): 698-702.
[12] 徐 徽, 苏元智, 李新海, 等. 盐湖水氯镁石制取轻质氧化镁工艺[J]. 中国有色金属学报, 2004, 14(10): 1776-1781
XU Hui, SU Yuan-zhi, LI Xin-hai, et al. Technology of preparation for light magnesium oxide from bischofite[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(10): 1776-1781.
[13] 杨兆娟, 向 兰. 从盐湖卤水中提锂的研究进展[J]. 海湖盐与化工, 2005, 34(6): 27-29.
YANG Zhao-juan, XIANG Lan. Progress on the extraction of lithium from the salt lake brine[J]. Sea-Lake Salt & Chemical Industry, 2005, 34(6): 27-29.
[14] 袁俊生, 纪志永. 海水提锂研究进展[J]. 海湖盐与化工, 2003, 32(5): 29-33.
YUAN Jun-sheng, JI Zhi-yong. The progress of extracting lithium from seawater[J]. Sea-Lake Salt & Chemical Industry, 2003, 32(5): 29-33.
[15] Mehta, Vijay C. Process for recovery lithium from saltbrines: US, 4723962[P]. 1988-02-09.
收稿日期:2008-01-06;修回日期:2008-02-25
基金项目:国家科技攻关计划资助项目(2005BA639C)
通信作者:徐 徽(1963-),男,湖南华容人,从事盐湖资源开发研究;电话:0731-8877352;E-mail: xuhui_0318@hotmail.com