
Electrochemical performance of multiphase nickel hydroxide
LUO Fang-chen(罗方承)1, 2, CHEN Qi-yuan(陈启元)1,YIN Zhou-lan(尹周澜)1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Jiangxi Kingan High-Tech Co. Ltd, Nanchang 330508, China
Received 6 March 2006; accepted 28 June 2006
Abstract: The high density nano-crystalline multiphase nickel hydroxide containing at least three doping elements was synthesized and its electrochemical characteristics were studied. The electrochemical behavior of the high density spherical multiphases α-Ni(OH)2 were also investigated. The results show that the structure of the material is a mixed phase of α-Ni(OH)2 and b-Ni(OH)2, which has a the same stabilized structure as α-Ni(OH)2 during long-term charge/discharge process. High density spherical multiphases α-Ni(OH)2 have a much better redox reversibility, a much lower oxidation potential of Ni(Ⅱ) than the corresponding oxidation state in the case of b-Ni(OH)2, and a much higher reduction potential. They exchange one electron during electrochemical reaction and have a higher proton diffusion coefficient. The mechanism of the electrode reaction is proton diffusion, and the proton diffusion coefficient is 5.67?10-10 cm2/s. Moreover, they reveal a higher discharge capacity than b-Ni(OH)2/b-NiOOH because they exchange one electron per nickel atom during charge/discharge process.
Key words: multiphase nickel hydroxide; electrochemical performance; voltammogram; exchange electron number; manganese
1 Introduction
Rechargeable batteries are critical components in exploding new technologies of portable electronics, power tools and electric vehicles. The Ni/Cd, Ni/MH, Ni/H2 and Ni/Zn batteries belong to alkaline secondary batteries. A common feature of these batteries is electrochemical active Ni(OH)2 electrode used as positive electrode and the Ni(OH)2 electrode is the essential limited factor in the overall battery energy density.
There are two polymorphs about the nickel hydroxide denoted α-Ni(OH)2 and b-Ni(OH)2. Both form crystals in the hexagonal system with the brucite-type structure with Ni(OH)2 layers stacked along the c-axis. The main difference between the b- and α-Ni(OH)2 phase resides in the stacking of the layers along the c-axis. For b-Ni(OH)2 layers are perfectly stacked along the c-axis with an interlamellar distance of 0.46 nm; while for α-Ni(OH)2 layers are completely misoriented relative to each other corresponding to a turbostratic phase in the presence of water molecules and anionic species at the van der WAALS gap. The interlamellar distance for the α-Ni(OH)2 is about 0.78 nm[1-6].
Conventional electrode reaction of b-Ni(OH)2 is considered to be a one-electron process involving oxidation of divalent nickel hydroxide to trivalent nickel oxyhydroxide in charge and subsequent discharge of trivalent nickel oxyhydroxide to divalent nickel hydroxide. Thus, b-Ni(OH)2 has a maximum theoretical specific capacity of 289 mA?h/g. However, it has also been reported that α-Ni(OH)2 has better electrochemical properties than b-Ni(OH)2[7-11]. α-Ni(OH)2 can be oxidized to γ-NiOOH at a lower potential than the corresponding oxidation state compared with b-Ni(OH)2, and has a higher discharge capacity than b-Ni(OH)2/ b-NiOOH since the valence of nickel in γ-NiOOH exceeds 3, due to Ni4+ defects (3.3-3.7)[12-13].
It is also reported that α-Ni(OH)2 is not stable in presence of the high concentrate basic electrolyte media and is transformed into b-Ni(OH)2. Attempts to improve stability of the α-Ni(OH)2 have a lot of reports[7-11]. In this paper, a high density multiphase α-Ni(OH)2 with stabilized α-Ni(OH)2/γ-NiOOH cycle during charge/ discharge process was synthesized through adding at least three modifier elements. It can improve capacity, cycle life and electrochemical performance. And the electrochemical performances of the high density spherical multiphase α-Ni(OH)2 were investigated.
2 Experimental
The material was synthesized by coprecipitation of several metal ions from the aqueous solution. The resulting brownish precipitate was filtered by a laboratory suction filter and washed with distilled water until it was free from neutral salts, then dried at 80 ℃ to produce powder. The powder was subjected to chemical and X-ray diffraction analysis. The doped metal ions are most preferably Co, Zn and Mn. For comparison all samples had the same Co and Zn contents, and only Mn content was changed.
The electrochemical behavior of the spherical multiphase nickel hydroxide in 6 mol/L KOH electrolyte was studied. A typical three-electrode one-compartment electrolysis cell was used for the experimental measurements. The working electrode was a platinum powder microelectrode with diameter of 200 mm. Nickel sheet counter electrode was placed on the side and the working electrode was positioned at the center. A LUGGIN capillary with a salt bridge was used to connect the cell to the reference electrode. All potentials were measured and quoted with respect to a Ag/AgCl (3 mol/L NaCl) reference electrode.
Cyclic voltammetric experiments were performed using EG & G PAR Mode 273A potentiostat/galvanostat, and a computer interface for experimental control and automatic data acquisition was used.
3 Results and discussion
Fig.1 shows the typical cyclic voltammograms for multiphase nickel hydroxide microelectrode with various Mn contents, which was activated by 15 cyclic voltammograms at a rate of 5 mV/s. In the range of scanning potentials employed, an anode oxidation peak for the electrode with various Mn contents, appearing between 0.34 and 0.38 V, was recorded prior to oxygen evolution. One oxyhydroxide reduction peak at about 0.162-0.177 V was observed on the reverse sweep. As shown in Fig.1, the potential of oxidation peak that corresponds to the formation of higher-valence nickel species apparently decreases with the increase of Mn content in nickel hydroxide, while the potential of reduction peak changes a little. Similar voltammogram is also observed (Fig.1(a)) for the electrode without Mn. The anodic oxidation peak corresponding to Ni(Ⅱ) oxidation reaction shifts to more positive potentials, and cathodic peak potential corresponding to nickel hydroxide reduction reaction shifts to less positive compared to the characteristics of the above results. The cyclic voltammetric results in Fig.1 are listed in Table 1. To compare the effect of multiphase α-Ni(OH)2 on oxygen evolution reaction, the difference between the oxidation peak potential and the oxygen evolution potential(DOP) on the reverse sweep required to produce 50 μA of anodic current is also estimated from voltammograms.
The results in Fig.1 and Table 1 illustrate that multiphase α-Ni(OH)2 allows the electrode to charge at a significantly less positive potential (387, 352 and 346 mV) instead of 412 mV. In addition, the charge process occurs more reversibly (DEac is 210, 168 and 184 mV instead of 254 mV). Moreover, their oxygen evolution overpotentials have nearly the same values. Thus, these results clearly indicate that multiphase α-Ni(OH)2 electrodes make the charge process perform more easily and more reversibly, suggesting that much more active materials can be utilized during charge.
Table 1 Parameters for nickel hydroxide electrode with various mass fraction of Mn

From Figs.1-3, it can be seen that the nickel hydroxide microelectrodes with different Mn contents, which were activated by 15 cyclic voltammograms at a rate of 5 mV/s, have a single diffusion-limited peak vs Ag/AgCl. Theoretically, potential difference of peak to peak on a voltammogram for a reversible electrode reaction should be equal to 59 mV/n, however the values in Table 1 are markedly higher than the theoretical value. Therefore, electrode reaction of nickel hydroxide is usually considered as a quasi-reversible electrode reaction[12-14]. The dependence of Epa and Epa/2 can be expressed using Nicholson and Shain equation (Eqn.(1))[15] and can be used to calculate the number of electrons (n) participating in the rate determining stages:
Epa-Epa/2=1.857RT/(αnF) (1)
where Epa/2 is the potential of half peak height, R is gas constant, T is absolute temperature, α is transfer coefficient and F is faraday constant. According to Fig.1 and Eqn.(1), the number of electrons participating in electrode reaction for nickel hydroxide microelectrode can be calculated, and the results are listed in Table 2. In addition, since oxygen evolution is a parasitic reaction during charge of nickel electrode, it will obscure the basic line of reduction peak current. Fig.4 shows that the anodic peak current (Ipa) in Fig.2 depends linearly on the square root of the potential sweep rate. As seen in Fig.4, there exists a linear relationship between the peak current and the potential sweep rate square root.

Fig.1 Cyclic voltammograms of multiphase nickel hydroxide with various mass fractions of Mn at 15th charge/discharge cycle: (a) Without Mn; (b) 10 % Mn, 15 % Mn and 20% Mn

Fig.2 Cyclic voltammograms of 15% Mn electrode at various sweep rates
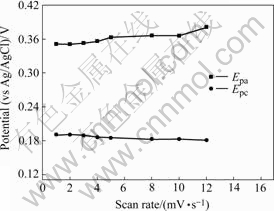
Fig.3 Effect of sweep rate on anodic and cathodic potentials for 15% Mn electrode
The charge-discharge process of the nickel hydroxide in alkaline solution can be described as a proton deintercalation-intercalation mechanism:

As a stationary electrode, a linear relationship between peak current and the square root of potential sweep rate (v1/2) over the range of sweep rate in Fig.2 can be expressed as
Ip = 2.99×105n(αn)1/2ADo1/2Coov1/2 (3)
where Do is the proton diffusion coefficient in nickel hydroxide, Coo is the concentration of hydrogen in the solid (assuming the density of Ni(OH)2 is 3.5 g/cm3 and thus the maximum concentration of electrochemically active material hydrogen in the lattice, Coo, is 0.038 3 mol/cm3[16]), and A is the area of electrode. According to Fig.2, Fig.4, Table 2 and Eqn.(3), diffusion coefficient of H+ for 15% Mn multiphase nickel hydroxide is found to be 6.67×10-10 cm2/s. Similarly, the others can also be calculated, the results are listed in Table 2.
Table 2 Diffusion coefficients of H+ for nickel hydroxide electrode with various mass fractions of Mn

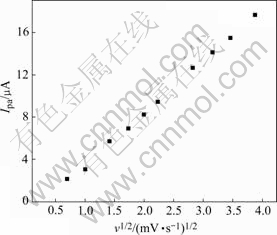
Fig.4 Dependence of anodic peak current on square root of potential sweep rate for 15% Mn electrode
In Table 2, the proton diffusion coefficients in the multiphase nickel hydroxide exhibit higher values, which are higher than those in Al-substituted α-Ni(OH)2, 3.6×10-11 cm2/s reported in Refs.[6-7].
As seen from Fig.1, Tables 1 and 2, the multiphase α-Ni(OH)2 doped with different amounts of Mn reveals much better electrochemical performance than Mn- undoped b-Ni(OH)2, such as much lower oxidation potential of nickel (Ⅱ), much higher electrochemical reversibility, and much more exchange electron number, thus the material has greater discharge capacity and higher utilization of active material.
The anions strongly anchor the positive charged brucite layers and stabilize the structure under a variety of stressful conditions. However, sample with lower anion content, namely that with 5% Mn is less stable in alkali media. As seen from Fig.1 and Table 2, the multiphase nickel hydroxide electrode that consists of 15% Mn has much better electrochemical performance, much more exchange electron number, and thus the electrode has greater discharge capacity than the theoretical capacity of b-Ni(OH)2 (289 mA?h/g). As a consequence, it can be concluded that α-Ni(OH)2 doped with 15% Mn is an excellent positive active material for alkaline rechargeable batteries.
4 Conclusions
1) Nickel hydroxide active materials internally containing at least three modifier elements, have a modified proton diffusion coefficient, excellent electrochemical performance and wider use of the available storage sites through improved proton transport.
2) The nickel hydroxide doped with 15% Mn has much lower oxidation peak potential and much higher reduction peak potential. The presence of Mn in nickel hydroxide lattice can increase electronic conductivity and protonic conductivity of nickel hydroxide, thus improving the performance of nickel hydroxide electrode, such as greater discharging capacity and better reversibility of Ni(Ⅱ)/Ni(Ⅲ) redox reaction.
References
[1] KOSTECKI R, MOLARNON F. Electrochemical and in situ Raman spectroscopic characterization of nickel hydroxide electrodes [J]. Journal of Electrochemical Society, 1997, 144(2): 485-493.
[2] KIM M S, HWANG T S, KIM K B. A study of the electrochemical redox behavior of electrochemically precipitated nickel hydroxides using electrochemical quartz crystal microbalance [J]. Journal of Electrochemical Society, 1997, 144(5): 1537-1543.
[3] RAMESH T N, VISHNU K P. Synthesis of nickel hydroxide: Effect of precipitation conditions on phase selectivity and structural disorder [J]. Journal of Power Sources, 2006, 156: 655-661.
[4] RAMESH T N, VISHNU KAMATH P, SHIVAKUMARA C. Correlation of structural disorder with the reversible discharge capacity of nickel hydroxide electrode [J]. Journal of Electrochemical Society, 2005, 152(4): A806-A810.
[5] MORGAN D, MENG Y S. Phase stability of nickel hydroxides and oxyhydroxides[J]. Journal of Electrochemical Society, 2006, 153(2): A210-A215.
[6] KAMATH P V, DIXIT M, INDIRA L, SHUKLA A K, LUMAR V G, MUNICHANDRAIAH N. Stabilized α-Ni(OH)2 as electrode material for alkaline secondary cells [J]. Journal of Electrochemical Society, 1994, 141(11): 2956-2959.
[7] DIXIT M, JAYASHREE R S, VISHNU K P. Electrochemically imprenated aluminum-stabilized α-nickel hydroxide electrodes [J]. Electrochemical and Solid-State Letters, 1999, 2: 170-171.
[8] EMOURGUES L, DELMAS C. Electrochemical behavior of the manganese-substituted nickel hydroxides[J]. Journal of Electro- chemical Society, 1996, 143: 561-566.
[9] HU W K, GAO X P, NOREUSD. Evaluation of nano-crystal sized α-nickel hydroxide as an electrode material for alkaline rechargeable cells [J]. Journal of Power Sources, 2006, 160: 704-710.
[10] DAI J X, SAM, LI Y. Structural stability of aluminum stabilized α nickel hydroxide as a positive electrode material for alkaline secondary batteries [J]. Journal of Power Sources, 2000, 89: 40-45.
[11] WANG X, LUO H, PARKHUTIK P V. Studies of the performance of nanostructural multiphase nickel hydroxide [J]. Journal of Power Sources, 2003, 115: 153-160.
[12] CORRIGAN D A, BENDERT R M. Effect of coprecipitated metal ions on the electrochemistry of nickel hydroxide thin films: Cyclic voltammetry in 1 mol/L KOH [J]. Journal of Electrochemical Society, 1989, 136(3): 723-728.
[13] WANG X Y, YAN J, YUAN T, ZHANG Y. Surface modification and electrochemical studies of spherical nickel hydroxide [J]. Journal of Power Sources, 1998, 72(2): 221-225.
[14] ZHANG C, PARK S M. The anodic oxidation of nickel in alkaline media studied by spectroelectrochemical techniques [J]. Journal of Electrochemical Society, 1987, 134(12): 2966-2970.
[15] Southampton Electro-chem group. Instrumental Methods in Electrochemistry [M]. New York: Ellis Horwood Ltd, 1990: 178-227.
[16] ZIMMERMAN A H, EFFA P K. Discharge kinetics of the nickel electrode [J]. Journal of Electrochemical Society, 1984, 131: 709-713.
Corresponding author: LUO Fang-chen; Tel: +86-791-3432146; E-mail: fcluo2003@yahoo.cn
(Edited by CHEN Wei-ping)