攀枝花钛铁矿的氢气还原动力学
来源期刊:中国有色金属学报(英文版)2016年第12期
论文作者:路长远 邹星礼 鲁雄刚 谢学良 郑凯 肖玮 程红伟 李光石
文章页码:3266 - 3273
关键词:攀枝花钛铁矿;合成钛铁矿;氢气还原;动力学;控速步骤;镁元素迁移
Key words:Panzhihua ilmenite; synthetic ilmenite; hydrogen reduction; kinetics; rate-controlling step; magnesium migration
摘 要:采用TG、XRD和SEM等分析手段,系统研究了900~1050 °C条件下攀枝花钛铁矿的氢气还原过程。结果表明:在900 °C恒温还原过程中,还原产物为铁和金红石,当温度高于1000 °C时,亚铁板钛矿开始形成。在还原过程中,元素镁会逐渐富集并影响金属化过程。同时,讨论了局部化学反应和相关的还原动力学过程,反应控速步骤为扩散过程。由计算可知,在所选实验条件下,氢气还原攀枝花钛铁矿的表观活化能为117.56 kJ/mol,高于合成钛铁矿还原过程中的表观活化能。
Abstract: The hydrogen reduction of Panzhihua ilmenite concentrate in the temperature range of 900-1050 °C was systematically investigated by thermogravimetric analysis (TG), X-ray diffraction (XRD) and scanning electron microscopy (SEM) methods. It was shown that the products of the Panzhihua ilmenite reduced at 900 °C were metallic iron and rutile. Above 1000 °C, ferrous pseudobrookite solid solution was generated. During the reduction process, element Mg gradually concentrated to form Mg-rich zone which can influence the metallization process. The reduction reaction proceeded topochemically and its related reduction kinetics were also discussed. The kinetics of the reduction indicated that the rate-controlling step was the diffusion process. The apparent activation energy of the hydrogen reduction of Panzhihua ilmenite was calculated to be 117.56 kJ/mol, which was larger than that of synthetic ilmenite under the same reduction condition.
Trans. Nonferrous Met. Soc. China 26(2016) 3266-3273
Chang-yuan LU1,2,3, Xing-li ZOU1,2,3, Xiong-gang LU1,2,3, Xue-liang XIE1,2,3, Kai ZHENG1,2,3, Wei XIAO4, Hong-wei CHENG1,2,3, Guang-shi LI1,2,3
1. School of Materials Science and Engineering, Shanghai University, Shanghai 200072, China;
2. State Key Laboratory of Advanced Special Steel, Shanghai University, Shanghai 200072, China;
3. Shanghai Key Laboratory of Advanced Ferrometallurgy, Shanghai University, Shanghai 200072, China;
4. Shanghai Shanshan Tech. Co., Ltd., Shanghai 201209, China
Received 2 December 2015; accepted 30 August 2016
Abstract: The hydrogen reduction of Panzhihua ilmenite concentrate in the temperature range of 900-1050 °C was systematically investigated by thermogravimetric analysis (TG), X-ray diffraction (XRD) and scanning electron microscopy (SEM) methods. It was shown that the products of the Panzhihua ilmenite reduced at 900 °C were metallic iron and rutile. Above 1000 °C, ferrous pseudobrookite solid solution was generated. During the reduction process, element Mg gradually concentrated to form Mg-rich zone which can influence the metallization process. The reduction reaction proceeded topochemically and its related reduction kinetics were also discussed. The kinetics of the reduction indicated that the rate-controlling step was the diffusion process. The apparent activation energy of the hydrogen reduction of Panzhihua ilmenite was calculated to be 117.56 kJ/mol, which was larger than that of synthetic ilmenite under the same reduction condition.
Key words: Panzhihua ilmenite; synthetic ilmenite; hydrogen reduction; kinetics; rate-controlling step; magnesium migration
1 Introduction
The mineral ilmenite (nominally FeTiO3) is one of the primary mineral resources for producing titanium dioxide and Ti [1-3]. Panzhihua ilmenite in Sichuan, southwest of China, is one of the largest ilmenite reserves over the world with an estimated ilmenite reserve of about 8.7×108 t, which accounts for more than 90% of the total titanium resource of China and over 35% of the world [4-6]. Panzhihua ilmenite concentrate is a rock-type mineral that contains low-grade titania and high content of impurities (especially high content of MgO), which make it difficult to upgrade the ilmenite ore [7]. Due to its high impurity content, the reducibility of ilmenite is low and Panzhihua ilmenite is unsuitable for the chlorination process to produce TiO2 pigment [8,9]. In a traditional method, ilmenite ore is smelted with carbon in electric furnace for preparing titanium-bearing slag. The smelting process always requires a long time and a high temperature, and the slag-forming reagents added to produce a fluid titania- rich slag will dilute the concentration of titanium dioxide in the slag and have deleterious effects on the subsequent processes of extracting titania [10,11]. Therefore, it would be beneficial to develop a direct reduction process that produces solid titania-rich slag and metallic iron, and the metallic iron can be removed from the reduced products by either leaching or mechanical separation.
In recent decades, there has a rising interesting in the direct reduction of ilmenite ores, and hydrogen has been investigated as the predominant reductant for the direct reduction of ilmenite ores [12-15]. Furthermore, many studies on the reduction of synthetic ilmenite ores by hydrogen were reported. ZHAO and SHADMAN [16] as well as VIJAY et al [17] examined the reduction process of synthetic ilmenite by hydrogen. It was suggested that intrinsic chemical reaction and diffusion of gaseous species through product layer were the rate- controlling factors during the reduction process. The temporal profiles of conversion had a sigmoid shape and presented three different stages, i.e., original induction stage, medium acceleration stage, and final deceleration stage. VRIES et al [18,19] employed a pressurized thermogravimetric microbalance to investigate the reduction of synthetic ilmenite pellets in the temperature range of 550-900 °C and pressure range of (1.2-10)×105 Pa. However, these isothermal experiments of reduction of Bama ilmenite using H2-Ar gas mixtures were carried out by WANG et al [20].
However, the kinetics of reduction of Panzhihua ilmenite by hydrogen is still unclear because of high content of impurities in the ilmenite. In the present work, the influences of temperature and hydrogen content on the reduction rate and degree were investigated, and the reduction kinetics was also discussed. The activation energy of reduction reaction was calculated, and the hydrogen reduction mechanism of ilmenite concentrate was discussed based on the experimental results.
2 Experimental
2.1 Materials
The chemical composition of Panzhihua ilmenite concentrate used in the present work is shown in Table 1. For comparison, synthetic ilmenite was prepared from the mixture of Fe, Fe2O3, and TiO2 with an appropriate molar ratio at 1200 °C by roasting for 30 h. Figure 1 further indicates the crystalline phases of the synthetic ilmenite and Panzhihua ilmenite. As shown in Fig. 1, the main crystalline phases of the natural ilmenite are magnesian ilmenite ((Fe,Mg)TiO3) with a small amount of titanomagnetite (Fe2TiO4-Fe3O4). The natural ilmenite and synthetic ilmenite powders were ground by planetary ball mill and screened to obtain similar particle size fractions in the range of 26-104 μm. Then, approximately 1.2 g ilmenite powder was pressed into a cylindrical pellet sized 8 mm in diameter and 5 mm in thickness.
Table 1 Chemical composition of Panzhihua ilmenite (mass fraction, %)

2.2 Experimental procedure
2.2.1 Thermogravimetric (TG) analysis
The schematic diagram of the thermogravimetric apparatus for the hydrogen reduction experiments is shown in Fig. 2. It consists of a vertical furnace and a computer monitor system used for recording the mass variations during the process. In a typical experiment, an alumina crucible was hung on the central section of furnace chamber by a sapphire extension wire. The sample was put into the alumina crucible and then preheated to a specific temperature in argon atmosphere. Then, in the isothermal period, the reactant gases (H2-Ar mixture) were blown into the reaction area. Both of hydrogen and argon gases were measured and controlled by high-accuracy mass flow-meters. At the end of the experiment, pure argon gas was purged into the crucible. Finally, the sample was cooled in inert atmosphere.

Fig. 1 XRD patterns of Panzhihua natural ilmenite (a) and synthetic ilmenite (b)

Fig. 2 Schematic diagram of TG apparatus
2.2.2 Characterization of reduced ilmenite
The morphology of the reduced pellet was examined using a JEOL JSM-6700F scanning electron microscope (SEM). The elemental composition was analyzed by energy-dispersive X-ray (EDX) spectroscopy attached to the SEM and also by inductively coupled plasma (ICP). The phase constitution was determined by Rigaku D/Max-2550 X-ray diffractometer (XRD).
3 Results and discussion
As the gas product during the hydrogen reduction process is only water vapor, the reduction degree can be calculated based on the total mass loss during the reduction. Therefore, the experimental results are presented in the form of the mass loss of sample against reduction time. The mass loss fraction is defined as
 (1)
(1)
where m0 is the mass of the initial sample, mt is the mass of sample after reduction time t, and mcal is the mass loss obtained by theoretical calculation.
3.1 Effect of hydrogen content on reduction process
The Panzhihua ilmenite pellets were reduced at 1000 °C with 10%H2-Ar, 30%H2-Ar, and 50%H2-Ar mixture (volume fraction) gas, respectively. For each experiment, the temporal profile of conversion was determined by monitoring the sample mass using a recording electrobalance. As shown in Fig. 3, an increase in H2 content results in an increase in the reduction rate and a decrease in the time required to attain certain fractional mass loss.

Fig. 3 Reduction degrees of Panzhihua ilmenite concentrate reduced by H2-Ar mixture gas with various hydrogen contents at 1000 °C
3.2 Effect of reduction temperature on reduction process
Isothermal mass loss measurements were performed in the temperature range of 900-1050 °C. Figure 4 shows the influences of temperature on the reduction of Panzhihua ilmenite (Fig. 4(a)) and synthetic ilmenite (Fig. 4(b)) by H2-Ar mixture gas. The reduction rate and degree increase sharply when the temperature increases, and the reduction reaction can finish approximately at 900 °C for 200 min and at 1000 °C for 150 min with 50% H2-Ar mixture gas (volume fraction). The reduction rate of Panzhihua ilmenite is lower than that of the synthetic ilmenite, which may be attributed to the high content of impurities in the natural ilmenite. It is known that magnesium atom can dissolve in the ilmenite to form solid solution. Actually, the Panzhihua ilmenite can be expressed as (Fe,Mg)TiO3, which is a kind of solid solution mineral. Therefore, it is considered that the lower reduction rate of Panzhihua ilmenite may be mainly attributed to the magnesium oxide containing in the ilmenite.
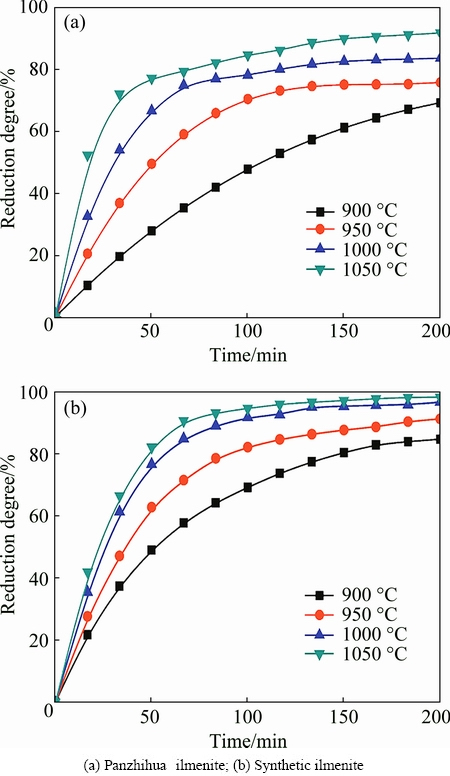
Fig. 4 Reduction degrees of ilmenite samples reduced by 50%H2-Ar mixture gas at different temperatures
The reduction of Panzhihua ilmenite and synthetic ilmenite using pure H2 was also investigated. As shown in Fig. 5(a), the reduction of Panzhihua ilmenite concentrate by using pure hydrogen at 900 °C for 200 min is incomplete and the reduction degree is approximately 70%. When the reduction temperature increases to 1000 °C, the reduction degree can reach about 92% within 200 min, which shows that higher temperature can improve the reduction rate. When the temperature is above 900 °C, the reaction proceeds rapidly in the initial stage. However, the reaction speed decreases after being reduced for 50 min. As shown in Fig. 5(b), the reduction rate of synthetic ilmenite by H2 is higher than that of Panzhihua ilmenite concentrate under the same conditions. The reduction degree approaches 98% at 1050 °C within 100 min.
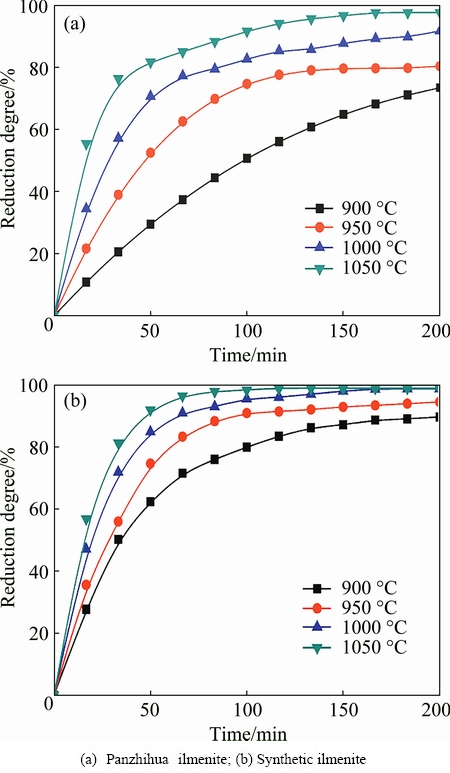
Fig. 5 Reduction degrees of ilmenite samples reduced by pure H2 at different temperatures
3.3 Phase transformation during reduction process
XRD analysis results of the reduced Panzhihua ilmenite samples are given in Fig. 6 and Table 2.
The phases of the sample reduced at 900 °C include iron, magnesian ilmenite and rutile. The diffraction peak intensities of ilmenite and magnesian ilmenite decrease apparently when iron phase appears. The magnesian ilmenite phase is not obvious in the XRD pattern of the sample reduced at 1000 °C. Moreover, with increasing the temperature, the peak intensity of rutile decreases evidently, which suggests that rutile can hardly generate from the solid-solution mineral as an independent phase above 1000°C. The increase in the peak intensity of ferrous pseudobrookite solid solution phase ((Fe,Mg)Ti2O5) indicates that M3O5 phases are formed, where M represents the combination of elements such as iron, magnesium and titanium. It was reported that the thermodynamic activity of iron could be lowered due to the formation of M3O5 solid solution [21]. The reported results indicated that the reduction of TiO2 may occur as long as iron metallization is completed [16,20]. However, the iron in Panzhihua ilmenite can hardly achieve complete metallization due to the formation of stable solid solution. Therefore, during the reduction process for ilmenite, the reduction of TiO2 is inhibited.

Fig. 6 XRD patterns of initial sample (a) and obtained samples reduced by pure H2 at 900 °C (b), 1000 °C (c) and 1050 °C (d) for 200 min
Table 2 Phase composition of Panzhihua ilmenite reduced by pure H2 at different temperatures for 200 min

3.4 Morphology of reduced ilmenite concentrate
Figure 7 shows the micromorphology of the initial Panzhihua ilmenite particle (Fig. 7(a)) and the ilmenite after being reduced at 1000 °C with pure hydrogen for 200 min (Fig. 7(b)). It can be seen that the initial Panzhihua ilmenite particle has a compact structure with no obvious gaps. And several small slivers can be observed on its surface. In contrast, a lot of pores are formed on the surfaces of the ilmenite particles reduced by pure hydrogen at 1000 °C (Fig. 7(b)). The formation of the porous structure may be attributed to the removal of oxygen component containing in the ilmenite particle during the reduction process.

Fig. 7 SEM images of Panzhihua ilmenite before (a) and after (b) being reduced by pure hydrogen at 1000 °C for 200 min

Fig. 8 Micromorphologies of cross section of Panzhihua ilmenite reduced by pure hydrogen at 1000 °C for 30 min (a), 60 min (b) and 90 min (c)
The reduced Panzhihua ilmenite samples embedded in epoxy resin were cut and polished. Figure 8 shows the cross section micromorphologies of the samples reduced by pure hydrogen at 1000 °C for different reduction time. Obviously, two phases are observed, the bright one is the metallic iron and the dark gray one may be attributed to the phase enriched in titania. EDX mapping images of Ti, Mg, O and Fe are also shown in Figs. 8(a), (b) and (c). It is worth noticing that the magnesium moves gradually and then forms an enrichment core. This phenomenon could be explained by the barrier effect [22]. During the reduction process, magnesium diffused ahead of the reaction interface to form a magnesium barrier zone in which Fe2+ was replaced by Mg2+, inhibited hydrogen diffusion in the pellet and hence the activity of Fe2+ was lower. The lower activity of the Fe2+ would make its reduction process more difficult. Figure 9 shows the schematic diagram showing the barrier effect of magnesium. Moreover, as magnesium oxide can form a more stable solid solution with titanium and iron oxides, magnesium oxide thus has larger barrier effect than other impurities such as silicon oxide and aluminium oxide.

Fig. 9 Schematic illustration of barrier effect of magnesium during reduction process
3.5 Reduction kinetics
Based on the results of thermogravimetric and micromorphology analyses, it can be concluded that the hydrogen reduction of ilmenite concentrate is a topochemical reaction. According to the reaction kinetics, the relationship between the reduction time (t) and reverse degree (R) can be expressed as [20]

 (2)
(2)
where C0 and Ceq are the hydrogen contents on ilmenite surface and in equilibrium, respectively, k is the reaction rate constant, De is the effective diffusion coefficient, r0 is the characteristic initial radius of ilmenite pellet, and ρ0 is the initial oxygen content in the ilmenite.
According to the solid-state kinetics [22-24], Eq. (3) is controlled by chemical reactions at the interface, Eq. (4) is controlled by diffusion, and Eq. (5) is controlled by both chemical reactions and diffusion.
1-(1-R)1/3=kt (3)
1-(2/3)R-(1-R)2/3=kt (4)
3k1[1-(1-R)1/3]+(3/2)k2[1-(2/3)R-(1-R)2/3]=t (5)
where k, k1 and k2 are the rate constants at different rate-controlling steps.
Based on the results shown in Figs. 4 and 5 and applying Eq. (2), three cases for rate controlling step were examined. It is found that the diffusion of hydrogen gas in the reduced layer was the rate controlling step under all experimental conditions owing to the better linear correlation of 1-(2/3)R-(1-R)2/3 to time. The relationship between 1-(2/3)R-(1-R)2/3 and time is shown in Figs. 10 and 11 for 50% H2 and pure H2 gas, respectively.

Fig. 10 Relationship between 1-(2/3)R-(1-R)2/3 and time for reduction by 50%H2-Ar mixture gas
Figure 12 shows the relationship between the calculated reaction rate constant and reciprocal temperature according to the Arrhenius equation. With the extra step of curve-fitting, it is found that the natural logarithm of reaction rate constant (ln k) is linearly associated with reciprocal temperature. The apparent activation energies of Panzhihua ilmenite concentrate reduced by 50% H2 and pure H2 are calculated to be 128.65 and 117.56 kJ/mol, respectively. And the apparent activation energies of synthetic ilmenite reduced by 50% H2 and pure H2 are determined to be 74.42 and 77.80 kJ/mol, respectively. Therefore, the reduction of Panzhihua ilmenite requires higher energy to cross the energy barrier than that for the synthetic ilmenite.

Fig. 11 Relationship between 1-(2/3)R-(1-R)2/3 and time for reduction by pure H2

Fig. 12 Calculated interface reaction rate constant as function of reciprocal temperature
4 Conclusions
1) In the temperature range of 900-1050 °C, the reduction speed and reduction degree of Panzhihua ilmenite and synthetic ilmenite by H2-Ar mixture gas increase with the increase of temperature and hydrogen content. The products of the Panzhihua ilmenite reduced at approximately 900 °C are metallic iron and rutile. Above 1000 °C, ferrous pseudobrookite solid solution is generated.
2) Compared with synthetic ilmenite, the reduction speed and reduction degree for Panzhihua ilmenite are relatively low due to the impurities containing in the ilmenite. During the reduction process, magnesium containing in the Panzhihua ilmenite can influence the reduction of iron.
3) The reductive kinetics is controlled by the hydrogen diffusion in the reduced layer under the experimental conditions. The apparent activation energy of the hydrogen reduction of Panzhihua ilmenite is evidently larger than that of the synthetic ilmenite. The apparent activation energies of Panzhihua ilmenite concentrate reduced by 50% H2 and pure H2 are calculated to be 128.65 and 117.56 kJ/mol, respectively.
References
[1] TAO Tao, CHEN Qi-yuan, HU Hui-ping, YIN Zhou-lan, CHEN Ying. TiO2 nanoparticles prepared by hydrochloric acid leaching of mechanically activated and carbothermic reduced ilmenite [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(5): 1232-1238.
[2] HUANG R, LV X W, BAI C G, DENG Q Y, MA S W. Solid state and smelting reduction of Panzhihua ilmenite concentrate with coke [J]. Canadian Metallurgical Quarterly, 2012, 51(4): 434-439.
[3] XIAO Wei, LU Xiong-gang, ZOU Xing-li, WEI Xue-mei, DING Wei-zhong. Phase transitions, micro-morphology and its oxidation mechanism in oxidation of ilmenite (FeTiO3) powder [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(8): 2439-2445.
[4] WU Fei-xiang, LI Xin-hai, WANG Zhi-xing, WU Ling, GUO Hua-jun, XIONG Xun-hui, ZHANG Xiao-ping, WANG Xiao-juan. Hydrogen peroxide leaching of hydrolyzed titania residue prepared from mechanically activated Panzhihua ilmenite leached by hydrochloric acid [J]. International Journal of Mineral Processing, 2011, 98(1): 106-112.
[5] LIU Shui-shi, GUO Yu-feng, QIU Guan-zhou, JIANG Tao, CHEN Feng. Solid-state reduction kinetics and mechanism of pre-oxidized vanadium–titanium magnetite concentrate [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(10): 3372-3377.
[6] LI Chun, LIANG Bin, GUO Ling-hong, WU Zi-bin. Effect of mechanical activation on the dissolution of Panzhihua ilmenite [J]. Minerals Engineering, 2006, 19(14): 1430-1438.
[7] LIU Chen-hui, ZHANG Li-bo, PENG Jin-hui, LIU Bing-guo, XIA Hong-ying, GU Xiao-chun, SHI Yi-feng. Effect of temperature on dielectric property and microwave heating behavior of low grade Panzhihua ilmenite ore [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(11): 3462-3469.
[8] SAMANTA S, MUKHERJEE S, DEY R. Oxidation behaviour and phase characterization of titaniferous magnetite ore of eastern India [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(9): 2976-2985.
[9] CHEN De-sheng, ZHAO Long-sheng, LIU Ya-hui, QI Tao, WANG Jian-chong, WANG Li-na. A novel process for recovery of iron, titanium, and vanadium from titanomagnetite concentrates: NaOH molten salt roasting and water leaching processes [J]. Journal of Hazardous Materials, 2013, 244: 588-595.
[10] Ostrovski O, Zhang G, Kononov R, DEWAN M A R, LI J. Carbothermal solid state reduction of stable metal oxides [J]. Steel Research International, 2010, 81(10): 841-846.
[11] DANG Jie, ZHANG Guo-hua, CHOU Kuo-chih. Kinetics and mechanism of hydrogen reduction of ilmenite powders [J]. Journal of Alloys and Compounds, 2015, 619: 443-451.
[12] WELHAM N J. Novel process for enhanced lunar oxygen recovery [J]. Journal of Materials Science, 2001, 36(9): 2343-2348.
[13] Gupta S K, Rajakumar V, Grieveson P. Kinetics of reduction of ilmenite with graphite at 1000 to 1100 °C [J]. Metallurgical Transactions B, 1987, 18(4): 713-718.
[14] Park E, Ostrovski O. Reduction of titania-ferrous ore by hydrogen [J]. ISIJ International, 2004, 44(6): 999-1005.
[15] CHEN Min, TANG Ai-tao, XIAO Xuan. Effect of milling time on carbothermic reduction of ilmenite [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4201-4206.
[16] ZHAO Y, SHADMAN F. Reduction of ilmenite with hydrogen [J]. Industrial & Engineering Chemistry Research, 1991, 30(9): 2080-2087.
[17] VIJAY P L, VENUGOPALAN R, SATHIYAMOORTHY D. Preoxidation and hydrogen reduction of ilmenite in a fluidized bed reactor [J]. Metallurgical and Materials Transactions B, 1996, 27(5): 731-738.
[18] de VRIES M L, GREY I E. Influence of pressure on the kinetics of synthetic llmenite reduction in hydrogen [J]. Metallurgical and Materials Transactions B, 2006, 37(2): 199-208.
[19] de VRIES M L, GREY I E, GERALD J D F. Crystallographic control in ilmenite reduction [J]. Metallurgical and Materials Transactions B, 2007, 38(2): 267-277.
[20] WANG Y M, YUAN Z F, MATSUURA H, TSUKIHASHI F. Reduction extraction kinetics of titania and iron from an ilmenite by H2-Ar gas mixtures [J]. ISIJ International, 2009, 49(2): 164-170.
[21] GUPTA S K, GRIEVESON P. Reduction behavior of ilmenite with carbon at 1240 °C [J]. Metallurgical and Materials Transactions B, 1995, 26(2): 401-404.
[22] WANG Yu-ming, YUAN Zhang-fu. Reductive kinetics of the reaction between a natural ilmenite and carbon [J]. International Journal of Mineral Processing, 2006, 81(3): 133-140.
[23] SI Xin-guo, LU Xiong-gang, LI Chuan-wei, LI Chong-he, Ding Wei-zhong. Phase transformation and reduction kinetics during the hydrogen reduction of ilmenite concentrate [J]. International Journal of Minerals, Metallurgy and Materials, 2012, 19(5): 384-390.
[24] El-Tawil S Z, Morsi I M, Francis A A. Kinetics of solid-state reduction of ilmenite ore [J]. Canadian Metallurgical Quarterly, 1993, 32(4): 281-288.
路长远1,2,3,邹星礼1,2,3,鲁雄刚1,2,3,谢学良1,2,3,郑 凯1,2,3,肖 玮4,程红伟1,2,3,李光石1,2,3
1. 上海大学 材料科学与工程学院,上海 200072;
2. 上海大学 省部共建高品质特殊钢冶金与制备国家重点实验室,上海 200072;
3. 上海大学 上海市钢铁冶金新技术开发应用重点实验室, 上海 200072;
4. 上海杉杉科技有限公司, 上海 201209
摘 要:采用TG、XRD和SEM等分析手段,系统研究了900~1050 °C条件下攀枝花钛铁矿的氢气还原过程。结果表明:在900 °C恒温还原过程中,还原产物为铁和金红石,当温度高于1000 °C时,亚铁板钛矿开始形成。在还原过程中,元素镁会逐渐富集并影响金属化过程。同时,讨论了局部化学反应和相关的还原动力学过程,反应控速步骤为扩散过程。由计算可知,在所选实验条件下,氢气还原攀枝花钛铁矿的表观活化能为117.56 kJ/mol,高于合成钛铁矿还原过程中的表观活化能。
关键词:攀枝花钛铁矿;合成钛铁矿;氢气还原;动力学;控速步骤;镁元素迁移
(Edited by Wei-ping CHEN)
Foundation item: Project (2014CB643403) supported by the National Basic Research Program of China; Projects (51225401, 51304132, 51574164) supported by the National Natural Science Foundation of China; Project (14JC1491400) supported by the Science and Technology Commissions of Shanghai Municipality, China; Project (2013GZ0146) supported by the Sichuan Province, China
Corresponding author: Xiong-gang LU; Tel/Fax: +86-21-56335768; E-mail: luxg@shu.edu.cn
DOI: 10.1016/S1003-6326(16)64460-6

