文章编号:1004-0609(2013)12-3410-13
尖晶石锰酸锂卤水提锂热力学
司秀芬1,张伟光1,何利华1,梁新星1,赵中伟1, 2
(1. 中南大学 冶金与环境学院,长沙 410083;
2. 中南大学 稀有金属冶金与材料制备湖南省重点实验室,长沙 410083)
摘 要:针对高Mg和Li质量比盐湖卤水提锂难的问题,提出利用尖晶石LiMn2O4对盐湖卤水进行选择性提锂,并在热力学计算的基础上绘制了298.15 K时Me(Li,Na,K,Mg)-Mn-H2O体系的φ—pH图,讨论尖晶石LiMn2O4脱Li+后所形成的λ-MnO2对盐湖中Na+、K+、Mg2+与Li+的选择性提取问题。结果表明:当离子浓度为1 mmol/L,体系阴极极化电位降至0.79 V(vs SHE)时,λ-MnO2中Mn4+被还原为Mn3+,同时溶液中Li+由于“记忆效应”而嵌入λ-MnO2晶格生成LiMn2O4;而极化电位需降至0.61、0.55和0.48 V时,才分别有Mg0.5Mn2O4、KMn2O4和 NaMn2O4生成,说明所选材料对Li+的选择性优于对Na+、K+和Mg2+的选择性。此外,根据西台吉乃尔盐湖卤水中主要组成阳离子浓度([Li]=30 mmol/L,[Na]=5 mol/L,[K]=0.2 mol/L,[Mg]=0.5 mol/L)绘制Me(Li,Na,K,Mg)-Mn-H2O系叠加φ—pH图。热力学研究表明:只需在卤水原始pH条件下将体系的阴极极化电位调至0.70 V<φ<0.87 V,λ-MnO2即可实现对Li+与大量Na+、Mg2+、K+的有效分离;将嵌Li+后的LiMn2O4通过调节电位极化至φ>0.87 V,可实现Li+的脱附和富集。
关键词:Me-Mn-H2O系;LiMn2O4;热力学;卤水提锂
中图分类号:TQ 021.2 文献标志码:A
Thermodynamics of Li-extraction from brine using spinel LiMn2O4
SI Xiu-fen1, ZHANG Wei-guang1, HE Li-hua1, LIANG Xin-xing1, ZHAO Zhong-wei1, 2
(1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. Hunan Key Laboratory for Metallurgy and Material Processing of Rare Metals, Central South University, Changsha 410083, China)
Abstract: The spinel LiMn2O4 was chosen for extracting Li+ from brine with high mass ratio of Mg and Li. The corresponding φ—pH diagrams of Me (Li, Na, K, Mg)-Mn-H2O systems at 298.15 K were plotted and analyzed according to thermodynamic calculation when the concentration of Me (Li, Na, K, Mg) was set as 1 mmol/L. The results show that when the redox potential of Li-Mn-H2O system is controlled less than 0.79 V (vs SHE), Mn4+ in λ-MnO2 crystal structure can be reduced to Mn3+, meanwhile, Li+ in solution can be inserted into λ-MnO2 crystal lattice due to memory effect to form LiMn2O4. However, the redox potential for Mg0.5Mn2O4, KMn2O4 and NaMn2O4 is 0.61, 0.55 and 0.48 V, respectively. It indicates that Li+ is prior to be inserted into λ-MnO2 from solution under the same condition compared with Na+, K+ and Mg2+. In addition, based on the main cation compositions ([Li]=30 mmo/L, [Na]=5 mol/L, [K]=0.2 mol/L, [Mg]=0.5 mol/L) in West Taijnar Salt Lake brine, the φ—pH diagrams were overlapped. The thermodynamics analysis shows that, in natural brine, it is possible that Li+ can be extracted effectively using λ-MnO2 when the potential is controlled between 0.70 V and 0.87 V. After that, the formed LiMn2O4 can release Li+ when a voltage 0.87 V is applied. By this means, Li+ in brine can be extracted and concentrated.
Key words: Me-Mn-H2O system; LiMn2O4; thermodynamics; Li extraction from brine
自1990年Sony公司将锂离子电池商业化以来,锂离子电池的高比能密度及良好的循环性能,使得锂在现代化工业中的地位迅速提升,将成为未来战略金属。锂资源主要以矿物锂和液态锂赋存于自然界中,其中液态锂约占85%[1-2],主要是海水、地表卤水及盐湖卤水,通常由Li+、Na+、K+、Mg2+、Ca2+等离子的氯化物、硫酸盐及碳酸盐组成。只是不同区域的卤水,其离子含量会有所不同[3-4]。通常认为[5-6],某盐湖是否具有开采价值,其中的Li+含量及Mg、Li质量比是决定因素。如果Li+含量太低,那么不具备开采价值;而如果Mg2+含量太高,就会限制经典沉淀法的应用,从而加长提取流程,大大提高开采成本。国外的低Mg、Li质量比盐湖卤水提锂已经工业化,我国盐湖卤水中蕴藏着丰富的锂资源,但由于卤水中Mg、Li质量比高,因此,科学家们采取各种方法从这种高Mg、Li质量比盐湖中提取锂,如结晶、沉淀、溶剂萃取及离子交换吸附法[7-11],但都未能实现工业化生产。
近年来,人们把从这种高Mg、Li质量比盐湖卤水提锂研究重点转向离子记忆无机离子交换剂,钛系和锰系锂离子筛是公认最有前途的提锂材料。钛氧化物离子筛具有化学稳定性好、力学强度高、吸附容量大、溶损度小的优点,但循环性能较差[12-13]。锂锰氧化物是最典型的具有尖晶石结构的λ-MnO2离子筛,因其价格低廉、无污染、对环境友好且对锂离子有高度选择性等特点而具有较好的应用前景。其操作首先是将“目标离子”(如Li)嵌入到λ-MnO2晶格中形成LiMn2O4,在不改变尖晶石结构的情况下进行热处理,然后再用HCl进行酸化处理,用H+置换出其中的Li,以形成锂空缺,这些空缺对“目标离子”(Li)有“记忆效应”,因此对“目标离子”有特殊的选择性,从而可以从卤水中吸附提取锂。从上述可以看出,锰系离子筛提锂的原理是通过调节溶液的pH值。然而,由于酸的作用和固体表面Mn3+的歧化反应,锰氧离子筛以Mn2+的形式溶解,导致循环性能下降[14-16]。
自20世纪以来,LiMn2O4在有机液锂离子电池中被普遍用作正极材料。而在1994年,为了解决锂离子电池用有机溶液作电解液易产生枝晶而容易引起爆炸等问题,DAHN等[17]的课题组在Science上开创性地提出水溶液锂离子电池。他们选择具有合适锂嵌脱电位的材料LiMn2O4、VO2作为电池正、负极,用5 mol/L LiNO3+1 mmol/L LiOH的水溶液(Li+的赋存状态类似于卤水中)作电解液,实验结果证明这样组装的水溶液锂离子电池具有一定的循环性能。随后,LI等[18]指出,也可以用价格低廉的LiMn2O4作两极材料,因为Li+在相对Li金属电位为4 V时能从LiMn2O4中脱出形成Li1-xMn2O4,而在相对Li金属电位为3 V时嵌入LiMn2O4形成Li1+xMn2O4,因而能产生1 V的电位差,如图1所示。且锰及其氧化物比Ni、Cd和Co,价格低廉,资源丰富,毒性较低。因此,他们认为Li1-xMn2O4/Li1+xMn2O4应当具有巨大的市场潜力。事实上,LiMn2O4在脱锂形成Li1-xMn2O4过程中,当x=1时,Li+从尖晶石骨架LiMn2O4中完全脱出后,其实就是λ-MnO2。而在LiMn2O4嵌锂形成Li1+xMn2O4的过程中,当x=1时,就形成层状LiMnO2。因此,LiMn2O4在脱嵌锂形成Li1-xMn2O4/Li1+xMn2O4的过程中,就是λ-MnO2(Li1-xMn2O4) LiMn2O4
LiMn2O4 LiMnO2(Li1+xMn2O4)之间转换。
LiMnO2(Li1+xMn2O4)之间转换。
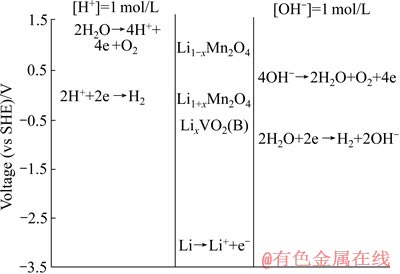
图1 当[Li+]=1.0 mol/L时在1 mol/L酸和1 mol/L碱溶液中各电极相对标准氢电极的电位图
Fig. 1 Potentials (vs SHE) in solutions with 1 mol/L H+ or 1 mol/L OH- at [Li+]=1.0 mol/L
同时可以看出,不同于锰氧离子筛通过控制pH值嵌脱锂,LiMn2O4是通过施加电位使其中的过渡金属锰离子发生氧化还原反应而实现嵌脱锂:当充电时,施加正电位,LiMn2O4中Mn3+会被氧化为Mn4+而释放一个电子,迫使相应的锂离子从LiMn2O4中分离出来,通过电解液移至负极,而与外电路过来的电子结合嵌入负极中,此时的LiMn2O4处于缺锂状态,为λ-MnO2(Li1-xMn2O4);而放电时,情况刚好相反,λ-MnO2(Li1-xMn2O4)中的Mn4+被还原为Mn3+,溶液中的Li+作为平衡离子嵌入λ-MnO2(Li1-xMn2O4)晶格中形成LiMn2O4(富锂状态)。因此,首先,用卤水代替水溶液锂离子电池中的电解液,以LiMn2O4及其脱锂后的λ-MnO2(Li1-xMn2O4)做电极,通过控制电位首先使卤水中Li+嵌入λ-MnO2(Li1-xMn2O4)中形成LiMn2O4,然后再对形成的LiMn2O4施加氧化电位,使其释放出Li+。重复上述过程可实现卤水中锂的分离与富集。
然而,由于实际卤水成分复杂,溶液中存在大量的Na+、Mg2+、K+等离子,尤其是Mg2+,它和Li+在元素周期表中处于对角线位置,化学性质相似。本文作者在文献[19]的基础上,拟通过对相应的Me(Li,Na,K,Mg)-Mn-H2O系进行热力学分析,从理论上深入了解LiMn2O4对各种离子提取的趋势,寻找LiMn2O4选择性提锂的可能性,以期为工艺试验提供理论指导。另外,为了讨论问题的方便,本研究中与LiMn2O4发生脱嵌Li+转换的λ-MnO2,均用MnO2替代。
1 Me(Li,Na,K,Mg)-Mn-H2O体系热力学计算
1.1 NaMn2O4标准生成吉布斯自由能的估算
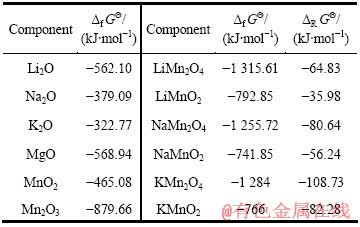
在298.15 K下,Li-Mn-H2O系中可以直接查得的稳定化合物或离子的热力学参数见表1。以LiMn2O4及 LiMnO2热力学数据为依据,利用在化学中广泛应用的同系线性规律对NaMn2O4及 NaMnO2的吉布斯自由能值进行估算。
根据同系线性规律,利用表1中锂、钠化合物的 数据,以锂化合物的
数据,以锂化合物的 为横坐标,钠化合物的
为横坐标,钠化合物的 为纵坐标进行线性拟合,其结果如图2所示。由图2可知,锂、钠化合物的
为纵坐标进行线性拟合,其结果如图2所示。由图2可知,锂、钠化合物的 数据几乎落在一条直线上,其线性方程为
数据几乎落在一条直线上,其线性方程为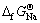 =0.983
=0.983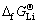 +37.12,线性相关系数R2=0.999。由此将LiMn2O4的
+37.12,线性相关系数R2=0.999。由此将LiMn2O4的 代入方程可求得NaMn2O4的
代入方程可求得NaMn2O4的 = -1 255.72 kJ/mol。将LiMnO2的
= -1 255.72 kJ/mol。将LiMnO2的 代入方程可求得NaMnO2的
代入方程可求得NaMnO2的 = -741.85 kJ/mol。
= -741.85 kJ/mol。
1.2 KMn2O4标准生成吉布斯自由能的估算
利用表1中钠、钾化合物的 数据,以钠化合物的
数据,以钠化合物的 为横坐标,钾化合物的Df GQ为纵坐标进行线性拟合,其结果如图3所示。由图3可知钠、钾化合物的
为横坐标,钾化合物的Df GQ为纵坐标进行线性拟合,其结果如图3所示。由图3可知钠、钾化合物的 数据几乎落在一条直线上,其线性方程为
数据几乎落在一条直线上,其线性方程为 =1.009
=1.009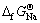 -8.436,线性相关系数R2=0.999。由此将NaMn2O4的
-8.436,线性相关系数R2=0.999。由此将NaMn2O4的 代入方程可求得KMn2O4的
代入方程可求得KMn2O4的 = -1 275 kJ/mol,将NaMnO2的
= -1 275 kJ/mol,将NaMnO2的 代入方程可求得KMnO2的
代入方程可求得KMnO2的 =-757 kJ/mol。
=-757 kJ/mol。
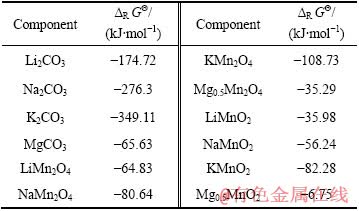
表1 Mn、Li、Na、K和Mg离子及其化合物的热力学数据
Table 1 Thermodynamic data of ion and compounds containing Mn, Li, Na, Mg and K
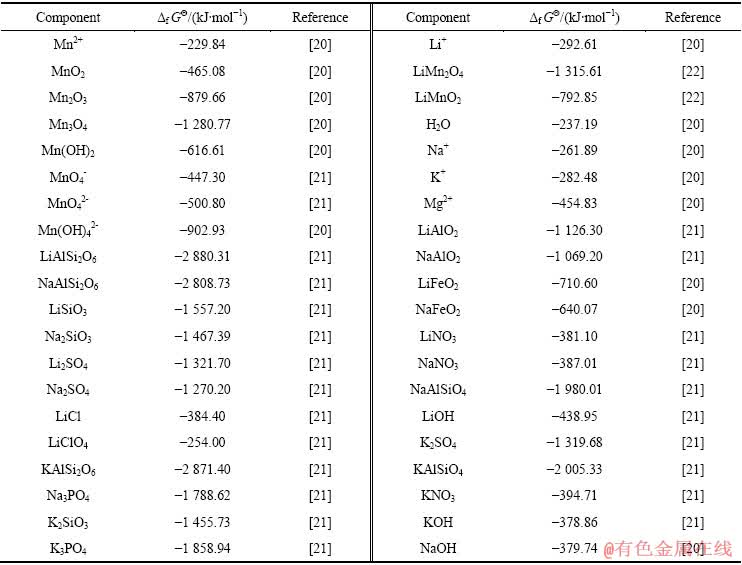
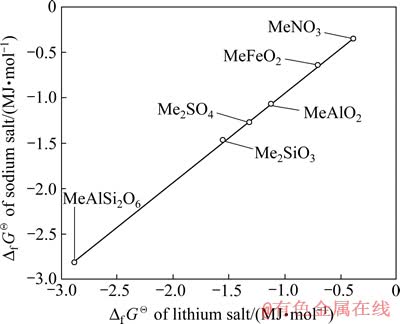
图2 Li盐与Na盐的Df GQ间的同系线性关系
Fig. 2 Linearity relation of Df GQ between lithium compounds and sodium compounds
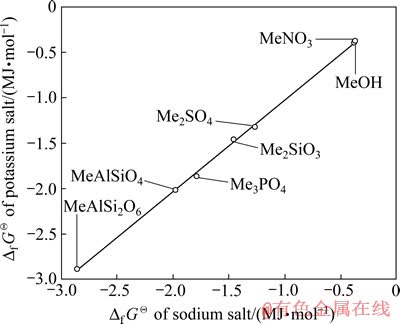
图3 Na盐与K盐的Df GQ间的同系线性关系
Fig. 3 Linearity relation of Df GQ between sodium compounds and potassium compounds
但考虑到这一数值是经过两步估算得到的,为检验其精度,再以锂的化合物来对钾的化合物进行估算。利用表1中锂、钾化合物的 数据,以锂化合物的
数据,以锂化合物的 为横坐标,钾化合物的
为横坐标,钾化合物的 为纵坐标进行线性拟合,其结果如图4所示。由图4可知,锂、钾化合物的
为纵坐标进行线性拟合,其结果如图4所示。由图4可知,锂、钾化合物的 数据也落在一条直线上,其线性方程为
数据也落在一条直线上,其线性方程为 =0.991
=0.991 +11.72,线性相关系数R2=0.998。由此将LiMn2O4的
+11.72,线性相关系数R2=0.998。由此将LiMn2O4的 代入方程可求得KMn2O4的Df GQ=-1 292 kJ/mol。将LiMnO2的
代入方程可求得KMn2O4的Df GQ=-1 292 kJ/mol。将LiMnO2的 代入方程可求得KMnO2的
代入方程可求得KMnO2的 = -774 kJ/mol。取两者平均值,即KMn2O4的
= -774 kJ/mol。取两者平均值,即KMn2O4的 = -1 284 kJ/mol;KMnO2的
= -1 284 kJ/mol;KMnO2的 =-766 kJ/mol。
=-766 kJ/mol。
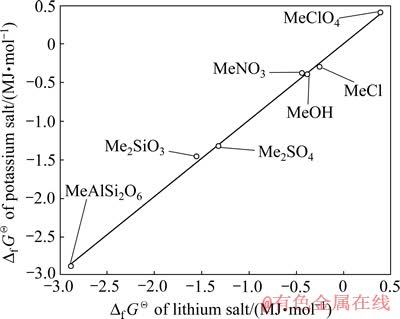
图4 Li盐与K盐的Df GQ间的同系线性关系
Fig. 4 Linearity relation of Df GQ between lithium compounds and potassium compounds
1.3 Mg0.5Mn2O4标准生成吉布斯自由能的估算
由于Mg0.5Mn2O4本身以及与镁元素同一主族化合物的相关热力学数据缺乏,因而不易采用同系线性规律对其 进行估算。在查阅大量文献基础上,本文作者对Mg0.5Mn2O4的
进行估算。在查阅大量文献基础上,本文作者对Mg0.5Mn2O4的 估算采用温元凯等[23]所述的复合氧化物反应自由能的线性相关性模型。根据温元凯等计算复杂含氧酸盐生成自由能的主体思想,复杂含氧酸盐可以看作是酸性氧化物、碱性氧化物的复合物,碱性最强的氧化物优先结合酸性最强的氧化物而形成含氧酸盐,然后,与酸性稍弱的氧化物复合。任何含氧酸盐都可以看作是由二种或三种氧化物反应而生成的盐,写成通式:
估算采用温元凯等[23]所述的复合氧化物反应自由能的线性相关性模型。根据温元凯等计算复杂含氧酸盐生成自由能的主体思想,复杂含氧酸盐可以看作是酸性氧化物、碱性氧化物的复合物,碱性最强的氧化物优先结合酸性最强的氧化物而形成含氧酸盐,然后,与酸性稍弱的氧化物复合。任何含氧酸盐都可以看作是由二种或三种氧化物反应而生成的盐,写成通式:
MmO+YOn=MmYOn+1 (1)
同时,含氧酸盐也可以写成一种复合氧化物:
MmYOn+1=MmO×YOn (2)
根据热化学循环可知,形成含氧酸盐时的反应自由能 和含氧酸盐生成自由能
和含氧酸盐生成自由能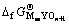 及始态氧化物生成自由能
及始态氧化物生成自由能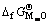 和
和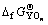 之间有如下关系:
之间有如下关系:
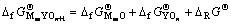 (3)
(3)
考察各类含氧酸盐的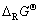 值,发现两种不同含氧酸盐系列的
值,发现两种不同含氧酸盐系列的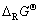 成线性关系。因此,利用此规律,分两步推算出Mg0.5Mn2O4的
成线性关系。因此,利用此规律,分两步推算出Mg0.5Mn2O4的 。首先,将LiMn2O4和LiMnO2拆分成相关氧化物,如图5所示,并在表2中列出相关数据,算出Li、Na、K相关锰酸盐的
。首先,将LiMn2O4和LiMnO2拆分成相关氧化物,如图5所示,并在表2中列出相关数据,算出Li、Na、K相关锰酸盐的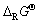 值。
值。
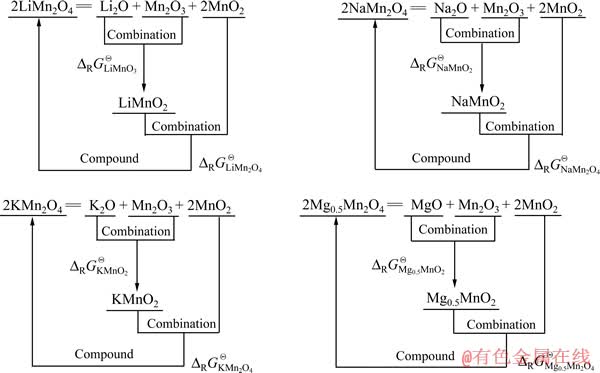
图5 Li、Na、K和Mg锰酸盐的拆分示意图
Fig. 5 Schematic diagrams of manganate for Li, Na, K and Mg divided into separate oxides
表2 Mn、Li、Na、K和Mg氧化物及其锰酸盐的热力学数据
Table 2 Thermodynamic data of ion and manganate compounds for Mn, Li, Na, K and Mg
根据文献[23]中公式:
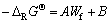 (4)
(4)
式中:Wf是离子的生成能参数;A、B是每一种含氧酸盐的常数。
推算出文献中所缺数据K2CO3的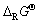 (K+的Wf=96,A=0.87,B=0),如表3所列。
(K+的Wf=96,A=0.87,B=0),如表3所列。
据此,利用两种不同含氧酸盐系列的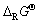 成线性关系,首先选取Li、Na、K碳酸盐的
成线性关系,首先选取Li、Na、K碳酸盐的 为横坐标(x),尖晶石锰酸盐的
为横坐标(x),尖晶石锰酸盐的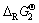 为纵坐标(y)进行线性拟合,求得线性方程为y=0.245x-19.21,R2=0.996。将MgCO3的
为纵坐标(y)进行线性拟合,求得线性方程为y=0.245x-19.21,R2=0.996。将MgCO3的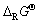 代入方程中,可求得Mg0.5Mn2O4的
代入方程中,可求得Mg0.5Mn2O4的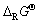 值。再以Li、Na、K尖晶石锰酸盐的
值。再以Li、Na、K尖晶石锰酸盐的 为横坐标(x),层状锰酸盐的
为横坐标(x),层状锰酸盐的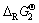 为纵坐标(y),进行线性拟合,求得线性方程为:y=1.039x+29.91,R2=0.992。将Mg0.5Mn2O4的
为纵坐标(y),进行线性拟合,求得线性方程为:y=1.039x+29.91,R2=0.992。将Mg0.5Mn2O4的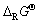 值代入方程,可求得Mg0.5MnO2的
值代入方程,可求得Mg0.5MnO2的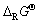 值,如表3所列。
值,如表3所列。
表3 Li、Na、K和Mg含氧酸盐的反应自由能
Table 3 Reaction free energy of oxysalt for Li, Na, K and Mg
再利用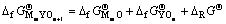 ,代入相关数据,可算出
,代入相关数据,可算出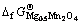 = -1 224.66 kJ/mol及
= -1 224.66 kJ/mol及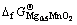 =-731.06 kJ/mol。
=-731.06 kJ/mol。
1.4 热力学平衡的计算
对于金属水系中可能存在的反应, 可以分为以下3种类型:
1) 无电子得失的水解-中和反应;
2) 有电子得失的氧化-还原反应;
3) 水解中和反应与氧化-还原反应共存。
第1)类反应pH值与其离子活度之间的关系通过平衡常数K求得,计算公式为
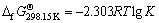 (5)
(5)
第2)类反应为半电池氧化-还原反应,化学反应方程式可表示为aA+bB+ze=cC+dD,其电位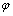 计算公式为
计算公式为
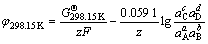 (6)
(6)
第3)类反应为氧化还原和水解共存的反应,其反应方程式可表示为aA+bB+ze+hH+=cC+dD,令H+的活度系数为1,则电位j的计算公式可表示为
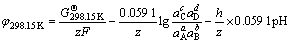 (7)
(7)
基于上述3 类反应计算方法以及Mn-H2O与Me(Li, Na, K, Mg)-Mn-H2O系中存在的反应, 在298.15 K以及O2和H2的分压均为101 325 Pa时, 求得体系中各平衡反应的φ—pH表达式, 如表4~8所列。
表4 Mn-H2O系中平衡反应在298.15 K下的反应式与φ—pH计算式
Table 4 φ—pH formulas of equilibrium reaction in Mn-H2O systems at 298.15 K
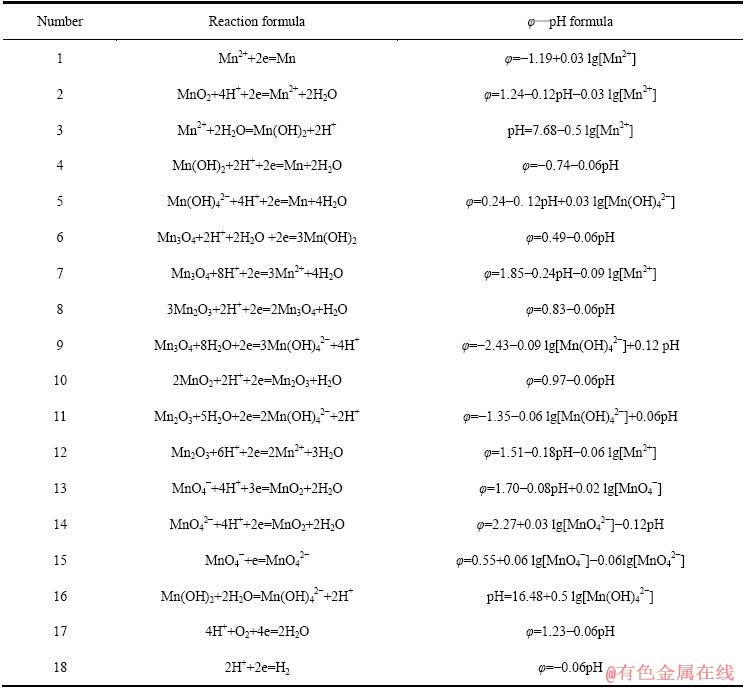
表5 Li-Mn-H2O系中平衡反应在298.15 K下的反应式与φ—pH计算式
Table 5 φ—pH formulas of equilibrium reaction in Li-Mn-H2O systems at 298.15 K
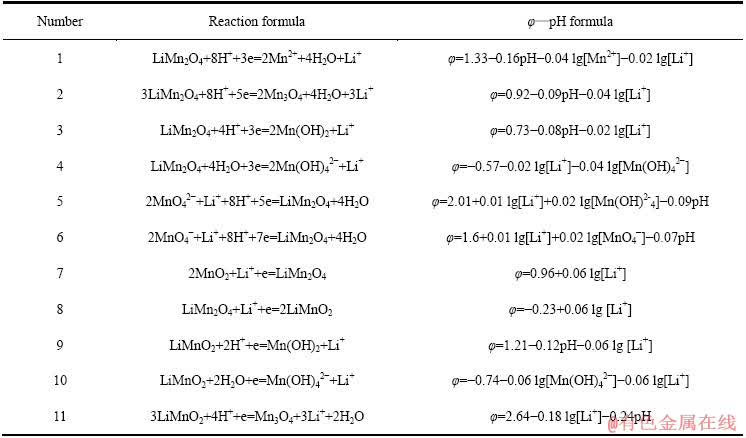
表6 Na-Mn-H2O系中平衡反应在298.15 K下的反应式与φ—pH计算式
Table 6 φ—pH formulas of equilibrium reaction in Na-Mn-H2O systems at 298.15 K
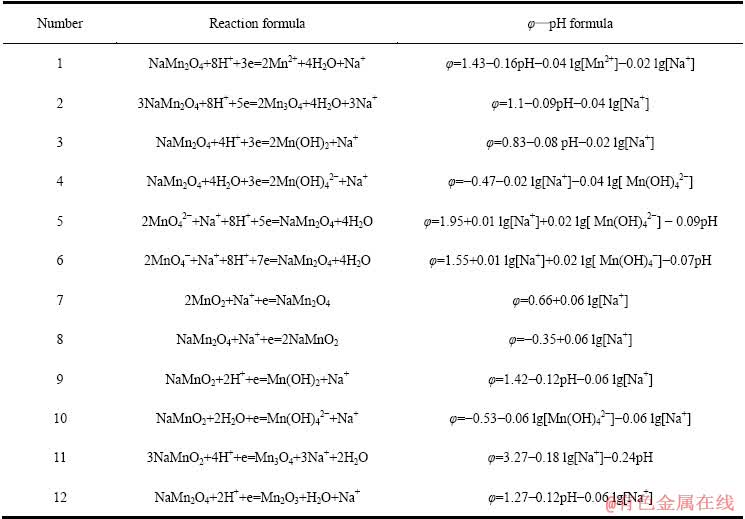
表7 K-Mn-H2O系中平衡反应在298.15 K下的反应式与φ—pH计算式
Table 7 φ—pH formulas of equilibrium reaction in K-Mn-H2O systems at 298.15 K
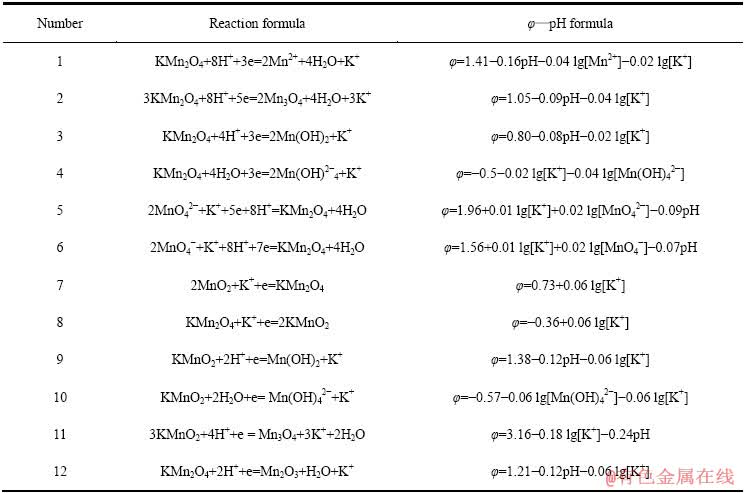
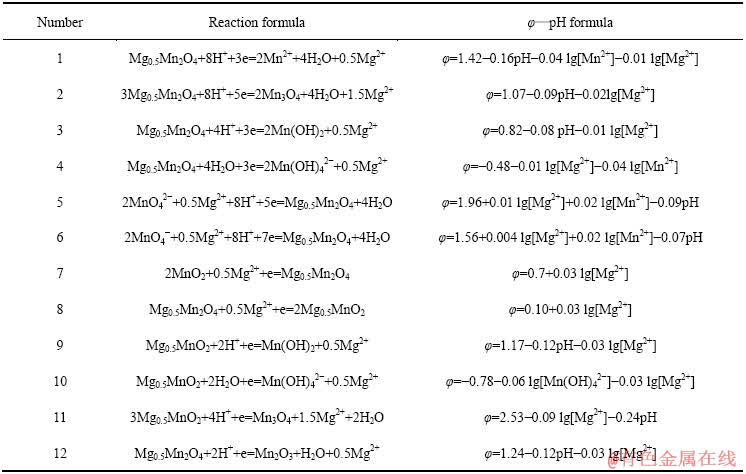
表8 Mg-Mn-H2O系中平衡反应在298.15 K下的反应式与φ—pH计算式
Table 8 φ—pH formulas of equilibrium reaction in Mg-Mn-H2O systems at 298.15 K
2 分析和讨论
利用尖晶石LiMn2O4进行卤水提锂,必须保证MnO2在水溶液中的稳定存在。赵中伟等[19]已绘绘制[Mn]=[Li]=1 mmol/L时的Mn-H2O系(图6)及Li-Mn- H2O系(图7)的φ—pH图。从图6可以看出,在水的稳定区内,锰的固相物质有Mn3O4和Mn2O3,部分MnO2及Mn(OH)2在水的稳定区内,而Mn则完全在水的稳定区外。在水的稳定区内,该体系在较低pH条件下锰主要以Mn2+的形态存在;而碱性条件下,则为Mn2O3、Mn3O4、Mn(OH)2及 的稳定区。二价锰的离子或化合物的稳定区位于体系中的氧化还原电势较低的区域,随着电势的升高,逐渐变为三价及四价的离子和化合物的稳定区。
的稳定区。二价锰的离子或化合物的稳定区位于体系中的氧化还原电势较低的区域,随着电势的升高,逐渐变为三价及四价的离子和化合物的稳定区。
对比图6和7可知:当在Mn-H2O体系中引入Li+后,热力学计算条件下Mn2O3稳定区不再存在,而被更大稳定区域的LiMn2O4相取代;MnO2、Mn3O4、Mn(OH)2及Mn(OH)42-的稳定区也有较大部分被LiMn2O4侵占。由图7中的①线可知,pH值在4.59~10.85范围内,当体系阴极极化电位φ≤0.79 V时,MnO2中Mn4+将被还原为Mn3+,由于体系中存在Li+,Li+会嵌入MnO2晶格而生成LiMn2O4。Li+嵌入MnO2的原因,大井健太等[24]认为,LiMn2O4中的锰有3、4两个价态(Li[Mn(Ⅲ)Mn(Ⅳ)O4),当Li+被脱出时得到保持着尖晶石结构的锰氧化物(λ-MnO2),此时锰发生氧化,形成四面体型空隙。该四面体空隙对Li+显示出较高的选择性,也就是在脱出Li+时的尖晶石结构中原Li+的“空位”就变成为吸着Li+的部位,因而, Li+会嵌入MnO2晶格而生成LiMn2O4。同时,可以看到,在[Li]=1 mmol/L时,无层状LiMnO2的区域,说明在低Li+浓度下,无LiMnO2生成。
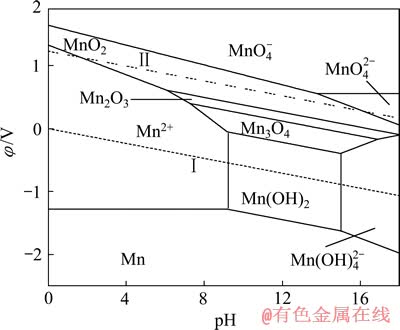
图6 Mn-H2O系的φ—pH图[19] ([Mn]=1 mmol/L)
Fig. 6 φ—pH diagram of Mn-H2O system[19] ([Mn]=1 mmol/L)
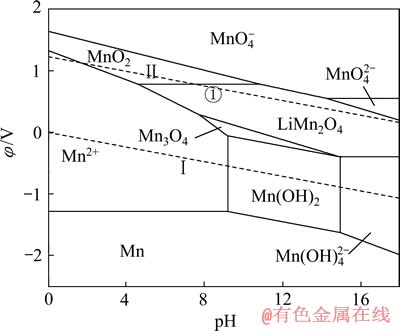
图7 Li-Mn-H2O系的φ—pH图[19] ([Li]=[Mn]=1 mmol/L)
Fig. 7 φ—pH diagram of Li-Mn-H2O system[19] ([Li]=[Mn]= 1 mmol/L)
盐湖卤水中存在大量Na+、K+和Mg2+,这些离子均可能对LiMn2O4选择性提锂造成干扰,因而有必要考察(Na、K、Mg)-Mn-H2O系。为考察LiMn2O4对Na+的吸附性能,现将离子浓度为1 mmol/L的Na+引入Mn-H2O体系中,可得到Na-Mn-H2O系,如图8所示。由图8可知,在Na-Mn-H2O系中,热力学上计算所得的Mn2O3稳定区由原始pH值为7.27~16.87的范围,缩小到pH值为7.27~10.55的较小区域,大部分被NaMn2O4吞噬。同时,NaMn2O4还吞噬了部分的MnO2和Mn3O4稳定区。由图8中②线可知,与Li+嵌入MnO2相似,当体系阴极极化电位φ≤0.48V、pH值为8.20~14.39时,可发生2MnO2+Na++e→NaMn2O4的反应。
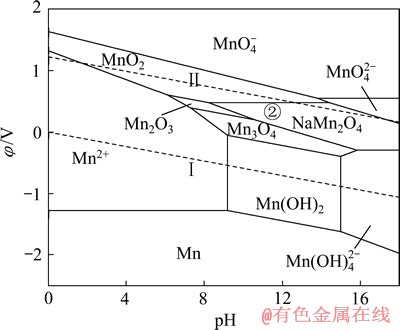
图8 Na-Mn-H2O系的φ—pH图 ([Na]=[Mn]=1 mmol/L)
Fig. 8 φ—pH diagram of Na-Mn-H2O system ([Na]=[Mn]=1 mmol/L)
同理,在上述Mn-H2O体系中引入1 mol/L的K+后,可得如图9所示的K-Mn-H2O系φ—pH图。与图8类似,在K-Mn-H2O系中,热力学上计算所得的Mn2O3稳定区由原始pH值为7.27~16.87的范围缩小到pH值为7.27~9.38的较小区域, 大部分被KMn2O4吞噬;同时,KMn2O4还吞噬了部分MnO2和Mn3O4的稳定区。由图9中的③线可知,当体系的阴极极化电位φ≤0.55 V、pH为7.02~13.80时,可发生 2MnO2+K++e→KMn2O4的反应。
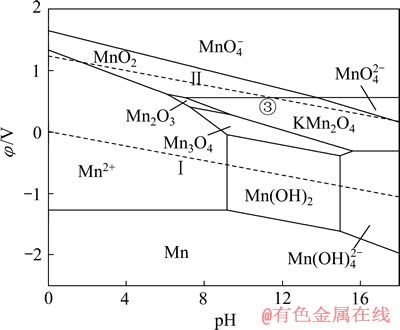
图9 K-Mn-H2O系的φ—pH图 ([K]=[Mn]=1 mmol/L)
Fig. 9 φ—pH diagram of K-Mn-H2O system ([K]=[Mn]=1 mmol/L)
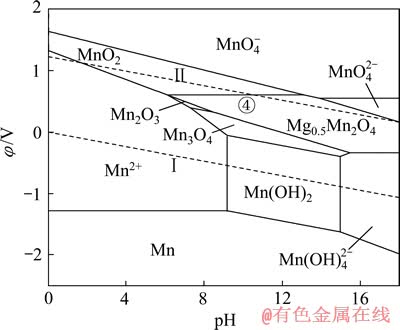
图10 Mg-Mn-H2O系的φ—pH图 ([Mg]=[Mn]=1 mmol/L)
Fig. 10 φ—pH diagram of Mg-Mn-H2O system ([Mg]= [Mn]=1 mmol/L)
相比Li、Na和Li、K,Li、Mg的分离是目前困扰盐湖界的一大难题。因此,继续在上述Mn-H2O体系中引入1 mmol/L的Mg2+后,可得如图10所示Mg-Mn-H2O系的φ—pH图。与图8、9类似,在Mg-Mn-H2O系中,热力学上计算所得的Mn2O3稳定区由原始pH值为7.27~16.87的范围,缩小到pH值 为7.27~8.45的较小区域,大部分被Mg0.5Mn2O4吞噬;同时,Mg0.5Mn2O4还吞噬了部分MnO2和Mn3O4的稳定区。由图10中的④线可知,当体系的阴极极化电位φ≤0.61 V,pH为6.09~13.11时,可发生2MnO2+0.5Mg2++e→Mg0.5Mn2O4的反应。
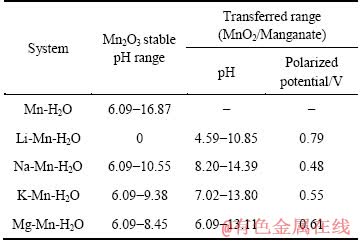
现将图6~10相关区域数据汇总至表9中。由表9可以看出,在Mn-H2O体系中分别引入相同离子浓度1 mmol/L的Li+、Na+、K+和Mg2+后,均出现了不同的尖晶石锰酸盐区域,Li+的最大,Mg2+的第二,K+的第三,Na+的最小,这和MnO2嵌Li+、Na+、K+和Mg2+的电位是相关的。当MnO2嵌入某离子发生还原时的电位(即阴极极化电位)相对越高,则形成该离子的锰酸盐稳定区域越大,说明对该离子的选择性越好,反之亦然。
表9 Li、Na、K、Mg φ—pH图中区域的对比
Table 9 Comparison of Li, Na, Mg and K areas in φ—pH diagram
以上热力学计算过程中,均设定Li+、Na+、K+和Mg2+离子浓度为1 mmol/L。然而实际盐湖卤水中Li+浓度较低,而Na+、Mg2+、K+浓度则为Li+浓度的几十甚至上百倍。为从热力学上考察LiMn2O4对实际盐湖卤水离子浓度的吸附情况,现以我国典型高Mg、Li质量比盐湖水—西台吉乃尔盐湖卤水中主要组成阳离子的浓度作参考[25],如表10所列,分别取[Li]=30 mmol/L,[Na]=5 mol/L,[K]=0.2 mol/L,[Mg]=0.5 mol/L,仍取[Mn]=1 mmol/L,进行热力学计算。
表10 西台吉乃尔盐湖地表湖水及晶间卤水部分组成[25]
Table 10 Partial compositions of salt lake brine and intercrystalline brine in West Taijnar[25]
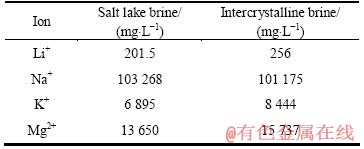
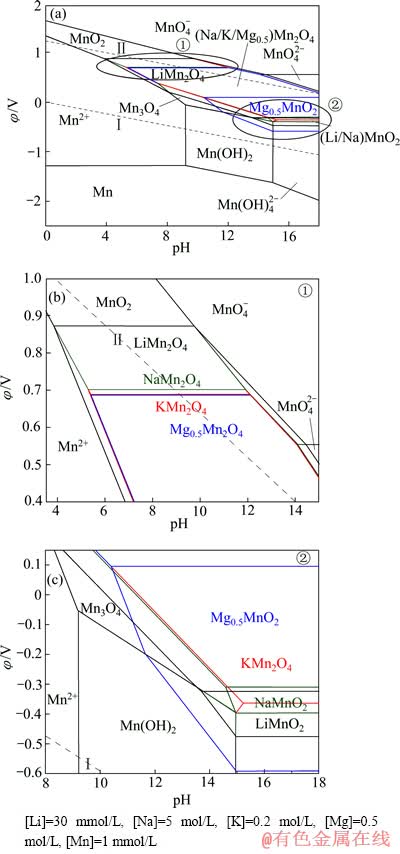
图11 Li-Mn-H2O系、Na-Mn-H2O系、K-Mn-H2O系和Mg-Mn-H2O系的φ—pH叠加图
Fig.11 φ—pH overlying diagrams of Li-Mn-H2O, Na-Mn-H2O, K-Mn-H2O and Mg-Mn-H2O systems ((Na/K/Mg0.5)Mn2O4 means combination of NaMn2O4, KMn2O4 and Mg0.5Mn2O4)
将所绘制的Li-Mn-H2O系、Na-Mn-H2O系、K-Mn-H2O系和Mg-Mn-H2O系的φ—pH图进行叠加,所得结果如图11(a)所示。由图11(a)可知,尽管Li+浓度最低,但LiMn2O4的稳定区依旧最大,而NaMn2O4、KMn2O4和Mg0.5Mn2O4仍占据相当区域,且除K+外,Li+、Na+和Mg2+均出现了层状LiMnO2、NaMnO2及Mg0.5MnO2的区域,且层状Mg0.5MnO2的区域最大。为了看得更清楚,现将11(a)中MnO2嵌Li+、Na+、K+和Mg2+离子形成尖晶石锰酸盐的区域①、形成层状锰酸盐的区域②放大,分别示于图11(b)和(c)中。
由图11(b)可见:热力学计算条件下在较高的阴极极化电位(φ=0.87 V)下就可以发生MnO2还原为LiMn2O4的反应,此时pH值范围为3.85~9.75;而由MnO2还原为NaMn2O4的极化电位φ=0.70 V,pH值范围为5.29~11.91;还原为KMn2O4的极化电位φ=0.688 V,pH值范围为5.41~12.07;还原为Mg0.5Mn2O4的极化电位φ=0.686 V,pH值范围为5.42~12.09。此外,在pH值范围为3.85~8.10的条件下,可发生Mn2+→LiMn2O4的反应;而NaMn2O4、KMn2O4与Mg0.5Mn2O4则不能由Mn2+直接生成,需要在更高氧化电位及pH条件下先生成Mn2O3或Mn3O4,经继续氧化而得到。同时还可看出,在高pH条件下,Li+、Na+、K+和Mg2+的尖晶石锰酸盐仍能稳定存在。由图11(c)可见,LiMn2O4、NaMn2O4与Mg0.5Mn2O4还原为LiMnO2、NaMnO2及Mg0.5MnO2的极化电位分别为-0.32 V、-0.31 V和0.10 V。可见,在形成层状锰酸盐的过程中,Mg2+仍然最具优势。
以上分析可知,在西台吉乃尔盐湖卤水中,尽管Na+和Mg2+的浓度比Li+的高出许多倍,LiMn2O4依旧对Li+有高度选择性,pH值范围为3.85~9.75。天然卤水pH值在7左右,由此推断,只需在天然卤水原始pH值条件下通过控制体系的阴极极化电位0.70 V< φ<0.87 V,即可实现MnO2从卤水中选择性嵌Li+形成LiMn2O4;将嵌Li+后的LiMn2O4通过调节体系的极化电位φ>0.87 V,即可发生LiMn2O4→MnO2+Li++e的脱附反应,不断循环该过程即可实现从卤水中选择性提锂的目的。
3 结论
1) 从热力学计算分析可知,用尖晶石LiMn2O4从溶液中提取Li+、Na+、K+和Mg2+时,LiMn2O4对Li+表现出良好的选择性:在Li、Na、K、Mg和Mn的离子浓度均为1 mmol/L的溶液中阴极极化时,离子嵌入λ-MnO2中由易到难的顺序为Li+、Mg2+、K+、Na+;当阴极电位微极化到0.79 V时,Li+就优先嵌入形成LiMn2O4;当电位进一步降低到0.61、0.55和0.48 V时,Mg2+、K+和Na+才分别嵌入。
2) 热力学计算表明:在Na+和Mg2+的浓度远高于Li+浓度的西台吉乃尔盐湖卤水中,Li+依然优先于其他金属离子嵌入。将极化电位调至0.70 V<φ<0.87 V 的范围,尖晶石λ-MnO2即可实现对Li+与大量Na+、Mg2+和K+的有效分离;将嵌Li+后的LiMn2O4通过调节极化电位至φ>0.87 V,可实现Li+的脱附和富集。
REFERENCES
[1] 潘立玲, 朱建华, 李渝渝. 锂资源及其开发技术进展[J]. 矿产综合利用, 2002, 4(2): 29-32.
PAN Li-ling, ZHU Jian-hua, LI Yu-yu. Lithium resources and the progress of their exploitation technique[J]. Multipurpose Utilization of Mineral Resource, 2002, 4(2): 29-32.
[2] 高 峰, 郑绵平, 乜 贞, 刘建华, 宋彭升. 盐湖卤水锂资源及其开发进展[J]. 地球学报, 2011, 32(4): 483-492.
GAO Feng, ZHENG Mian-ping, NIE Zhen, LIU Jian-hua, SONG Peng-sheng. Brine lithium resource in the salt lake and advances in its exploitation[J]. Acta Geoscientica Sinica, 2011, 32(4): 483-492.
[3] AVERILL W A, OLSON D L. A review of extractive processes for lithium from ores and brines[J]. Energy, 1978, 3: 305-313.
[4] 殷陶刚, 凤海元. 吸附法卤水提锂技术的研究进展[J]. 安徽化工, 2010, 36(4): 5-9.
YIN Tao-gang, FENG Hai-yuan. The study of adsorption techniques on the extraction of lithium from brines[J]. Anhui Chemical Industy, 2010, 36(4): 5-9.
[5] 袁俊生, 纪志永. 海水提锂研究进展[J]. 海湖盐与化工, 2003, 32(5): 29-33.
YUAN Jun-sheng, JI Zhi-yong. The progress of extracting lithium from seawater[J]. Sea-lake Salt and Chemical Industry, 2003, 32(5): 29-33.
[6] NIE Zhen, BU Ling-zhong, ZHENG Mian-ping, HUANG Wei-nong. Experimental study of natural brine solar ponds in Tibet[J]. Solar Energy, 2011, 85(7): 1537-1542.
[7] HAMZAOUI A H, JAMOUSSI B, M'NIF A. Lithium recovery from highly concentrated solutions: Response surface methodology (RSM) process parameters optimization[J]. Hydrometallurgy, 2008, 90(1): 1-7.
[8] JEROME L. Process for obtaining monohydrated lithium sulfate from natural brines: US, 6547836[P]. 2003-04-15.
[9]  E, HARRIS B. Properties of electrolyte solutions relevant to high concentration chloride leaching Ⅰ. Mixed aqueous solutions of hydrochloric acid and magnesium chloride[J]. Hydrometallurgy, 2008, 90: 177-191.
E, HARRIS B. Properties of electrolyte solutions relevant to high concentration chloride leaching Ⅰ. Mixed aqueous solutions of hydrochloric acid and magnesium chloride[J]. Hydrometallurgy, 2008, 90: 177-191.
[10] DEBERITZ J, KOEBELE K, SCHADE K. Method of separating NaCl from a LiCl solution: US, 6063345[P]. 2000-05-16.
[11] WEN Xian-ming, MA Pei-hua, ZHU Chao-liang, HE Qiong, DENG Xiao-chuan. Preliminary study on recovering lithium chloride from lithium-containing waters by nanofiltration[J]. Separation and Purification Technology, 2006, 49(3): 230-236.
[12] 钟 辉, 殷辉安. 偏钛酸型锂离子交换剂表面性质与选择吸附性研究[J]. 离子交换与吸附, 2003: 19(1): 55-60.
ZHONG Hui, YIN Hui-an. Study on the properties of the surface and absorpt of Li+ ion-exchanger of H2TiO3 type[J]. Exchange and Adsorption, 2003, 19(1): 55-60.
[13] 李骁龙, 李少鹏, 张钦辉, 于建国. TiO2 离子筛的合成及锂吸附性能研究[J]. 功能材料, 2009, 40(8): 1338-1341.
LI Xiao-long, LI Shao-peng, ZHANG Qin-hui, YU Jian-guo. Preparation and lithium adsorption of titania ion-sieves[J]. Journal of Functional Materials, 2009, 40(8): 1338-1341.
[14] MA Li-wen, CHEN Bai-zhen, SHI Xi-chang, ZHANG Wen, ZHANG Kun. Stability and Li+ extraction/adsorption properties of LiMxMn2-xO4(M=Ni, AI, Ti; 0≤x≤1) in aqueous solution[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 369(1/3): 88-94.
[15] 石西昌, 余亮良, 陈白珍, 张丽芬, 周定方. 尖晶石型锂离子筛的制备及其吸附性能[J]. 中南大学学报: 自然科学版, 2011, 42(8): 2198-2203.
SHI Xi-chang, YU Liang-liang, CHEN Bai-zhen, ZHANG Li-fen, ZHOU Ding-fang. Preparation and adsorption property of spinel-type lithium ion-sieve[J]. Journal of Central South University: Science and Technology, 2011, 42(8): 2198-2203.
[16] 陈白珍, 马立文, 石西昌, 徐 徽, 杨喜云. 锂离子筛前驱体制备方法的研究进展[J]. 无机盐工业, 2009, 41(7): 1-4.
CHEN Bai-zhen, MA Li-wen, SHI Xi-chang, XU Hui, YANG Xi-yun. Research progress on preparation methods for precursors of lithium ion-sieve[J]. Inorganic Chemicals Industry, 2009, 41(7): 1-4.
[17] LI W, DAHN J R, WAINWRIGHT D S. Rechargeable lithium batteries with aqueous electrolytes[J]. Science, 1994, 264: 1115-1117.
[18] LI W, DAHN J R. Lithium-ion cells with aqueous electrolytes[J]. Journal of the Electrochemical Society, 1995, 142(6): 1742-1746.
[19] 赵中伟, 霍广生. Li-Mn-H2O系热力学分析[J]. 中国有色金属学报, 2004, 14(11): 1926-1933.
ZHAO Zhong-wei, HUO Guang-sheng. Thermodynamic analysis of Li-Mn-H2O system[J]. The Chinese Journal of Nonferrous Metals, 2004, 14(11): 1926-1933.
[20] 林传仙. 矿物及有关化合物热力学数据手册[M]. 北京: 科技出版社, 1985.
LIN Chuan-xian. The handbook of mineral and the related compounds thermodynamic data[M]. Beijing: Science and Technology Press, 1985.
[21] 伊赫桑·巴伦. 纯物质热化学数据手册[M]. 北京: 科学出版社, 2003.
BARIN I. Thermochemical date of pure substances[M]. Beijing: Science Press, 2003.
[22] YOKOKAWA H, SAKAI N, YAMAJI K, HORITA T, ISHIKAWA M. Thermodynamic determining factors of the positive electrode potential of lithium batteries[J]. Solid State Ionics, 1998, 113/115: 1-9.
[23] 温元凯, 邵 俊. 离子极化导论[M]. 合肥: 安徽教育出版社, 1985: 262-267.
WEN Yuan-kai, SHAO Jun. The theory of ionic polarization[M]. Hefei: Anhui Education Press, 1985: 262-267.
[24] 刘亦凡, 大井健太. 离子记忆无机离子交换体[J]. 离子交换与吸附, 1994, 10(3): 264-269.
LIU Yi-fan, KENTA O. Inorganic ion exchanger with ion-memory ability[J]. Ion Exchanger and Adsorption, 1994, 10(3): 264-269.
[25] 赵中伟, 梁新星, 刘旭恒, 何利华. 磷酸铁离子筛卤水提锂热力学分析[J]. 中国有色金属学报, 2013, 23(2): 559-567.
ZHAO Zhong-wei, LIANG Xin-xing, LIU Xu-heng, HE Li-hua. Thermodynamic analysis of Li-extraction from brine using the FePO4 ion-sieve[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(2): 559-567.
(编辑 李艳红)
基金项目:长沙市科技计划产学研金合作资金专项(K1205034-11)
收稿日期:2013-01-18;修订日期:2013-04-28
通信作者:赵中伟,教授,博士;电话:0731-88830476;E-mail: zhaozw@csu.edu.cn