文章编号: 1004-0609(2006)06-1070-06
LiF-NaF-KF-K2TiF6-KBF4熔体中
电沉积TiB2的阴极过程
李 军1, 李 冰2
(1. 上海工程技术大学 材料工程学院, 上海 201600; 2. 华东理工大学 资源与环境工程学院, 上海 200237)
摘 要: 采用循环伏安法研究了700℃时46.5LiF-11.5NaF-42KF (摩尔分数, %)熔盐中Ti(Ⅳ) (c(K2TiF6)=0.2mol/L)和B(Ⅲ) (c(KBF4)=0.4mol/L)在铂电极上的电化学还原机理, 计算了各还原步骤传递的电子数。 研究了LiF-NaF-KF-K2TiF6-KBF4 (c(K2TiF4)=0.3mol/L, c(KBF4)=0.3, 0.6, 0.9mol/L)熔盐中电化学合成TiB2的阴极过程机理。 结果表明: Ti(Ⅳ)的电化学还原为三步电荷传递反应, 且阴极过程近似可逆; B(Ⅲ)在铂电极上的电化学反应机理为简单的三电子一步反应, 阴极过程近似可逆; Ti(Ⅳ)和B(Ⅲ)可在同一电位下发生共沉积并反应生成TiB2。
关键词: 二硼化钛; 电化学还原; 熔盐 中图分类号: TQ134.11
文献标识码: A
Cathode process of titanium diboride electrodeposition in
LiF-NaF-KF-K2TiF6-KBF4 molten salt
LI Jun1, LI Bing2
(1. School of Materials Engineering,
Shanghai University of Engineering Science, Shanghai 201600, China;
2. School of Resource and Environment Engineering,
East China University of Science and Technology, Shanghai 200237, China)
Abstract: The mechanism of the electrochemical reduction of Ti(Ⅳ) (c(K2TiF6)=0.2mol/L) and B(Ⅲ) (c(KBF4)=0.4mol/L) on the platinum electrode in 46.5LiF-11.5NaF-42KF (mole fraction, %) molten salt at 700℃ were investigated by means of the cyclic voltammetry technique. The cathode process of titanium diboride (TiB2) formation was also studied on the platinum electrode in the 46.5LiF-11.5NaF-42KF-K2TiF6-KBF4 (c(K2TiF4)=0.3mol/L, c(KBF4)=0.3, 0.6, 0.9mol/L)molten salt at 700℃. It is found that the electrochemical reduction of Ti(Ⅳ) proceeds in three-step charge transfer, the cathodic process is quasi-reversible. The electrochemical reduction of B(Ⅲ) occurs in one-step charge transfer, the cathodic process is quasi-reversible. The electrolytic codeposition of Ti(Ⅳ) and B(Ⅲ) with the formation of TiB2 can be achieved.
Key words: titanium diboride; electrochemical reduction; molten salt
在铝电解工业中, 碳作为阴极内衬材料存在着一些难以克服的缺点: 如不能被铝液所浸润, 容易被电解质和铝液渗透而破损等。 为了取代碳阴极内衬, 人们不断努力寻找新型材料, 大量的研究表明TiB2是最理想的铝电解阴极内衬材料[1] , 这是因为TiB2不仅能被铝液良好浸润, 而且能够有效抵抗熔融电解质和铝液的侵蚀。 一些材料科学工作者已采用熔盐电沉积的方法在不同基体上制备了相对纯净、 表面平整、 厚度均匀的TiB2镀层[2-8]。 这种方法将TiB2的合成和涂层的制备两个步骤结合在一起, 简化了工艺并比较经济, 有望解决传统碳阴极所面临的不足。 目前国内和国外关于电化学合成TiB2惰性阴极工艺的研究比较多, 而阴极过程机理的研究比较少并且结果还不尽相同[9-12]。 在所选体系中研究钛离子和硼离子的电化学反应机理及TiB2的合成机理具有重要的意义。 这将关系到实验参数的选择, 从而影响电镀层的质量。 本文作者采用循环伏安法研究了700℃时46.5LiF-11.5NaF-42KF体系中铂电极上Ti(Ⅳ)和B(Ⅲ)的电化学反应机理以及TiB2的阴极电化学合成机理, 并且分析了Ti(Ⅳ)和B(Ⅲ)如何在同一电位下共沉积并反应生成TiB2, 以便为氟化物熔体中电化学合成金属陶瓷硼化物提供一些理论依据。
1 实验
支持电解质为三元共晶Flinak体系46.5LiF-11.5NaF-42KF(摩尔分数, %), 熔点454℃, 密度1.96g/cm3, LiF、 NaF和KF均为分析纯试剂。 电活性物质为K2TiF6 和KBF4, 均为市售分析纯试剂。 为了避免由H+, OH-、 O2-和其它金属杂质离子引起的副反应的干扰, 溶剂盐46.5LiF-11.5NaF-42KF预先经过严格的净化, 使其脱水并除去微量杂质。 具体过程为: 将熔盐盛在高纯石墨坩埚内(内径 70mm, 外径94mm, 内高60mm, 外高80mm, 纯度≥96%), 并放入不锈钢反应器中。 首先进行真空脱水, 从室温升温至200℃(升温之前真空度应小于5Pa), 此温度下真空脱水2h。 然后停止抽真空, 通入高纯氩气并继续升温至700℃。 在此温度下稳定半小时。 当盐熔化均匀后将材料为光谱纯石墨的阴阳极放入熔盐进行预电解, 尽量除去熔盐中微量杂质。 预电解时, 阴阳极间的电压控制在2V。 不同预电解时间下除杂后熔盐纯度通过循环伏安法来测定, 在此基础上, 选取适宜的预电解时间。
研究电极和参比电极均用纯度为99.5%直径为0.5mm的铂丝。 辅助电极为光谱纯石墨棒。 为了消除参比电极和研究电极间熔盐欧姆电势的影响, 两极应尽量靠近。
电化学测量在上海辰华仪器有限公司生产的CHI1100型电化学测试仪上进行。
2 熔盐的背底
图1所示为700℃时不同预电解时间下(0, 2, 5, 6h)46.5LiF-11.5NaF-42KF体系中得到的循环伏安曲线。 工作电极铂的有效面积为0.314cm2。
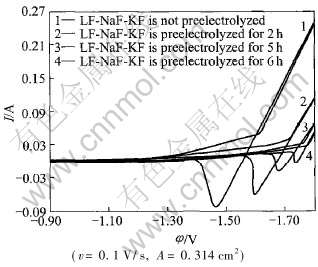
图1 46.5LiF-11.5NaF-42KF体系中
铂电极上的循环伏安曲线
Fig.1 Voltametric curves recorded on platinum electrode in system
46.5LiF-11.5NaF-42KF at 700℃
在预电解最初2h, 窗口电位和残余电流变化较大, 熔盐中杂质去除效果很明显。 但随着预电解时间的进一步延长, 窗口电位和残余电流变化逐渐减小, 表明除杂速率减慢。 尤其是预电解时间为5h和6h时, 窗口电位和残余电流变化已不很明显, 表明此时熔盐中杂质离子已经很少。 预电解时间达到6h时, 窗口电位为1.7790V, 残余电流为28.05mA。 当电位高于该窗口电位时, 电流急剧上升, 表明碱金属离子开始在阴极析出。 为了避免碱金属离子的析出干扰实验研究的准确性, 将预电解时间控制在6h, 并且实际窗口电位控制在1.5V。 此时, 残余电流大约为15mA, 几乎没有其它杂质离子或碱金属在铂电极上发生电化学反应。
3 Ti(Ⅳ)的电化学还原特征
实验表明, 对于被研究体系46.5LiF-11.5NaF-42KF-K2TiF6 (c(K2TiF6)=0.2mol/L), Ti(Ⅳ)在铂电极上反应的循环伏安图的基本特征都相同, 不同扫描速度下典型的循环伏安曲线见图2。

图2 46.5LiF-11.5NaF-42KF-K2TiF6 体系中
铂电极上得到的循环伏安曲线
Fig.2 Cyclic voltammograms recorded on platinum electrode in 46.5LiF-11.5NaF-42KF-K2TiF6 system at 700℃
循环曲线从初始电位0 V开始向负方向扫描, 当电位达到-1.5V时, 向正方向扫描, 电位重新达到0 V时, 扫描终止。 在负方向扫描中存在3个还原峰(a、 b、 c), 而在正方向扫描中存在3个相应的氧化峰(e、 f、 g)。 其中, 峰e、 f、 g是对应峰a、 b、 c的再氧化反应。 显然, Ti(Ⅳ)分三步还原为单质钛。 峰d的出现是最初还原形成的钛与电极铂形成合金发生溶解或吸附在电极表面上的钛离子发生脱附引起的。 当扫描速度从0.1到0.3V/s变化时, 各峰的峰电位变化不大。 利用峰电流(峰a、 b、 c)和扫描速度的平方根作图3, 均呈直线。 可以判定峰a、 b、 c对应的3个电化学反应为近似可逆反应[13]。

图3 峰电流和扫描速率平方根的关系
Fig.3 Peak current as function of scan rates
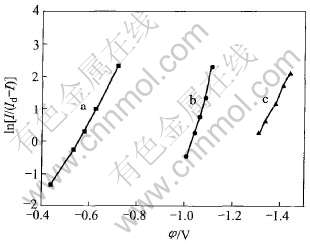
图4 ln[I/(Id-I)]和电位的关系
Fig.4 ln[I/(Id-I)] as function of potential E
本实验工作电极所用铂丝为圆柱形, 且其长度(l=2cm)远远大于半径(r=0.25mm)。 因此其扩散传质过程可以只考虑径向扩散作用, 电极横截面上的线性扩散作用可以忽略不计。 这就使得讨论该电极上的扩散电流性质更为方便。 基于这种假设, 推导出下面的电流和电位的关系[14, 15]:

根据方程(1)作曲线得到图4。 对第1个还原峰作φ和ln[I/(Id-I)]图, 得到直线a, 用最小二乘法处理数据后得该直线方程:
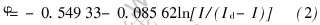
α=5%时, 线形回归显著。 由直线的斜率可求出电子转移数: n=0.979≈1。 因此第1个还原波a发生了一个电子转移, 对应反应为: 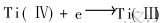 。
。
同理对第2、 第3个还原峰分别作φ和ln[I/(Id-I)]图, 得到直线b和c, 其直线方程为以及电子转移数分别为

α=5%时, 这两条直线线形回归显著。 因此第2个还原峰b和第3个还原峰c分别发生了两个电子和一个电子转移, 对应反应为: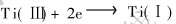 ,
,  。
。
4 B(Ⅲ)的电化学还原特征
实验结果表明, 对于被研究的体系46.5LiF-11.5NaF-42KF-KBF4 (c(KBF4)=0.4mol/L), B(Ⅲ)在铂电极上反应的循环伏安曲线的基本特征都相同。 不同扫描速度下典型的循环伏安曲线见图5。

图5 46.5LiF-11.5NaF-42KF-KBF4 体系中
铂电极上得到的循环伏安曲线
Fig.5 Cyclic voltammograms recorded on platinum electrode in 46.5LiF-11.5NaF-42KF-KBF4 system at 700℃
负方向扫描存在一个明显的还原峰a, 正方向扫描中存在一个相对应的氧化峰c, 初步说明B(Ⅲ)一步还原为单质硼。 利用峰电流和扫描速度的平方根作图6, 均得直线, 可以判定阴极过程近似可逆。
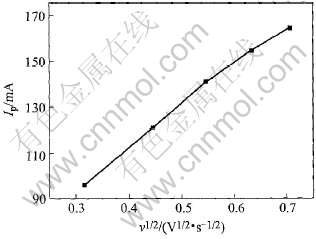
图6 峰电流和扫描速率平方根的关系
Fig.6 Peak current as function of square root of scan rates
同样根据式(1)作曲线得到φ和ln[I/(Id-I)]的关系(图 7)。 用最小二乘法处理数据后得到该直线方程为
φ=-0.86843-0.03254ln[I/(Id-I)](5)
α=5%时, 线形回归显著。 由直线的斜率可求出电子转移数: n≈3。 这也说明这一还原峰发生了3个电子转移, 对应反应为: 。
。

图7 ln[I/(Id-I)]和电位的关系
Fig.7 ln[I/(Id-I)] as function of potential φ
5 TiB2电沉积的阴极过程
图8所示为700℃, c(K2TiF4)=0.3mol/L, c(KBF4)分别为0.3, 0.6和0.9mol/L时的循环伏安曲线。 扫描速度从0.1V/s到0.5V/s时, 循环伏安曲线形状没有发生较大变化。 在负向扫描过程中出现2个还原峰(a, b), 而在正向扫描中出现2个相应的氧化峰(c, d)。 B/Ti从1到3时, 第2个峰的峰电位逐渐从113.15mV增加到139.03mV, 这主要是因为KBF4浓度的增加导致熔盐体系中活性离子供应充足的缘故。 第2个还原峰的初始还原电位都为1100mV。 在该电位下, 随熔盐中硼的增加, 电流变化不大, 约为80mA。 这说明电位小于1100mV时, B(Ⅲ)不会析出, 即, 第1个还原峰

图8 46.5LiF-11.5NaF-42KF-K2TiF6-KBF4 体系中铂电极上得到的循环伏安曲线
Fig.8 Cyclic voltammograms on platinum electrode in 46.5LiF-11.5NaF-42KF-K2TiF6-KBF4 system at 700℃
应该是Ti(Ⅳ)转变为低价钛离子所致。 比较图2和图8可知, 这个峰对应的反应是: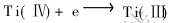 。 不难看出, 对于钛离子还原而言, 第2个还原峰是图2中Ti(Ⅲ)和Ti(Ⅰ)对应的第2个和第3个还原峰合并所致。 该峰和Ti(Ⅲ)对应的第2个还原峰的还原电位相近, 但比Ti(Ⅰ)对应的第3个还原峰的电位正了约300mV。 这说明在KBF4存在的情况下, Ti(Ⅲ)一步直接还原为单质钛。 比较图5和图8, 可知, B(Ⅲ)的还原电位向负移动了约240mV。 B(Ⅲ)的还原电位负移可归因于浓差极化的影响, 因为B(Ⅲ)的还原以及可能生成的TiB2都使阴极表面附近的浓度大大降低, 从而导致B(Ⅲ)的还原电位负移。 由上面的分析可知, 硼的沉积对Ti(Ⅰ)的还原产生了明显的去极化作用, 使得B(Ⅲ)和Ti(Ⅲ)在约-1100mV(相对于铂参比电极)电位下同时还原为单质。 由于TiB2的Gibbs生成自由能具有很高的负值(ΔGf, 1000K=-328.1kJ/mol), 它们将进一步反应生成TiB2[16]。 反应机理为
。 不难看出, 对于钛离子还原而言, 第2个还原峰是图2中Ti(Ⅲ)和Ti(Ⅰ)对应的第2个和第3个还原峰合并所致。 该峰和Ti(Ⅲ)对应的第2个还原峰的还原电位相近, 但比Ti(Ⅰ)对应的第3个还原峰的电位正了约300mV。 这说明在KBF4存在的情况下, Ti(Ⅲ)一步直接还原为单质钛。 比较图5和图8, 可知, B(Ⅲ)的还原电位向负移动了约240mV。 B(Ⅲ)的还原电位负移可归因于浓差极化的影响, 因为B(Ⅲ)的还原以及可能生成的TiB2都使阴极表面附近的浓度大大降低, 从而导致B(Ⅲ)的还原电位负移。 由上面的分析可知, 硼的沉积对Ti(Ⅰ)的还原产生了明显的去极化作用, 使得B(Ⅲ)和Ti(Ⅲ)在约-1100mV(相对于铂参比电极)电位下同时还原为单质。 由于TiB2的Gibbs生成自由能具有很高的负值(ΔGf, 1000K=-328.1kJ/mol), 它们将进一步反应生成TiB2[16]。 反应机理为

因此可以认为由于B(Ⅲ)的浓差极化和硼的沉积对Ti(Ⅰ)产生去极化作用促使硼和钛在同一电位下共沉积并反应生成TiB2。
6 结论
1) 700℃时46.5LiF-11.5NaF-42KF熔盐中Ti(Ⅳ) (c(K2TiF6)=0.2mol/L)在铂电极上电化学反应机理为三步反应, 反应如下: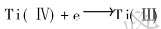 ,
,  ,
,  , 阴极过程近似可逆。
, 阴极过程近似可逆。
2) 700℃时46.5LiF-11.5NaF-42KF熔盐中B(Ⅲ) (c(KBF4)=0.4mol/L)在铂电极上的电化学反应机理为简单的三电子一步反应, 反应如下: , 阴极过程近似可逆。
, 阴极过程近似可逆。
3) 700℃时46.5LiF-11.5NaF-42KF-K2TiF6-KBF4 (c(K2TiF4)=0.3mol/L, c(KBF4)=0.3mol/L, 0.6mol/L, 0.9mol/L)熔盐中电化学合成TiB2的阴极过程机理为: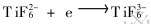 ,
, ,
, 。 B(Ⅲ)的极化和Ti(Ⅰ)的去极化促使硼和钛在同一电位下共沉积并反应生成TiB2: Ti+2B=TiB2。
。 B(Ⅲ)的极化和Ti(Ⅰ)的去极化促使硼和钛在同一电位下共沉积并反应生成TiB2: Ti+2B=TiB2。
REFERENCES
[1]Makyta M, Danek V, Haarberg G M, et al. Electrodeposition of titanium diboride from fused salts[J]. Journal of Applied Electrochemistry, 1996, 26: 319-324.
[2]王化章, 汤啸, 杨建红, 等. 氯化物熔体中电化学合成硼化钛[J]. 中国有色金属学报, 1997, 7(2): 34-37.
WANG Hua-zhang, TANG Xiao, YANG Jian-hong, et al. Electrochemical synthesis of TiB2 in chloride molten salts[J]. The Chinese Journal of Nonferrous Metals, 1997, 7(2): 34-37.
[3]李冰, 李军, 徐金富, 等. 在石墨基体上脉冲电镀TiB2镀层[J]. 稀有金属, 2004, 28(6): 1001-1005.
LI Bing, LI Jun, XU Jin-fu, et al. Pulse current plating of TiB2 coatings on graphite substrate[J]. Chinese Journal of Rare Metals, 2004, 28(6): 1001-1005.
[4]LI Jun, LI Bing, YE Yi-fu. Electrodeposition of titanium diboride from the KF-KCl molten salts[A]. Proceedings of the Second International Symposium on Metallurgy and Materials of Non-Ferrous Metals and Alloys[C]. Shenyang: Northeastern University, 2004. 8-12, 36-42.
[5]LI Jun, LI Bing, DONG Zhen-ge. Preparation of TiB2 coatings by electroplating in molten salt[J]. Rare Metals, 2005, 24(3): 261-266.
[6]LI Jun, LI Bing, DONG Zheng-ge. Electrodeposition of [001] oriented TiB2 coatings[J]. Materials Letters, 2005, 59: 3234-3237.
[7]Ett G, Pessine E J. Titanium diboride (TiB2) formation by electroplating in molten salt with continuous pulsed current[J]. Plating Surface Finishing, 2000. 118-120.
[8]Ett G, Elisabete J, Pessine E J. Pulse current plating of TiB2 in molten fluoride[J]. Electrochimica Acta, 1999, 44: 2859-2870.
[9]石青荣, 段淑贞, 赵立忠, 等. LiF-NaF-K2TiF6熔盐中Ti(Ⅳ)的电化学还原[J]. 腐蚀科学与防护技术, 1996, 8(3): 173-178.
SHI Qing-rong, DUAN Shu-zhen, ZHAO Li-zhong, et al. Electrochemical reduction of Ti(Ⅳ) in molten LiF-NaF-K2TiF6[J]. Corrosion Science and Protection Technology, 1996, 8(3): 173-178.
[10]石青荣, 段淑贞, 武士民. 氟化物熔盐中B(Ⅲ)的电化学还原[J]. 北京科技大学学报, 1994, 16(6): 599-603.
SHI Qing-rong, DUAN Shu-zhen, WU Shi-min. Electrochemical reduction of B(Ⅲ) in molten fluorides[J]. Journal of University Science and Technology Beijing, 1994, 16(6): 599-603.
[11]Makyta M, Matiasovsky K, Taranenko V I. Mechanism of the cathode process in the electrochemical synthesis of TiB2 in molten salts(Ⅰ)—The synthesis in an all-fluoride electrolyte[J]. Electrochim Acta, 1989, 34: 861-866.
[12]Taranenko V I, Zarutskii I V, Shapoval V I, et al. Mechanism of the cathode process in the electrochemical synthesis of TiB2 in molten salt(Ⅱ)—Chloride-fluoride electrolytes[J]. Electrochim Acta, 1992, 37: 263-268.
[13]段淑贞, 乔芝郁. 熔盐化学—原理和应用[M]. 北京: 冶金工业出版社, 1990. 220.
DUAN Shu-zhen, QIAO Zhi-yu. Molten Salt Chemistry—principle and Application[M]. Beijing: Metallurgical Industry Press, 1990. 220.
[14]蒋汉赢. 冶金电化学[M]. 北京: 冶金工业出版社, 1983. 110.
JIANG Han-yin. Metallurgical Electrochemistry[M]. Beijing: Metallurgical Industry Press, 1983. 110.
[15]张祖训, 汪尔康. 电化学原理和方法[M]. 北京: 科学出版社, 2000. 55.
ZHANG Zu-xun, WANG Er-kang. Electrochemistry Principle and Method[M]. Beijing: Science Press, 2000. 55.
[16]段淑贞, 石青荣, 王新东. 氟化物熔体中电解共沉积硼化钛的阴极过程[J]. 北京科技大学学报, 1996, 18(1): 46-49.
DUAN Shu-zhen, SHI Qing-rong, WANG Xin-dong. Cathode process in the electrolytic codeposition of titanium diboride in molten fluoride[J]. Journal of University Science and Technology Beijing, 1996, 18(1): 46-49.
基金项目: 国家自然科学基金资助项目(50204006)
收稿日期: 2005-10-27; 修订日期: 2006-03-13
通讯作者: 李 军, 博士; 电话: 021-67791203; E-mail: jacob_lijun@sina.com
(编辑陈爱华)