文章编号:1004-0609(2008)05-0862-05
添加剂Pr2O3和Er2O3对电沉积Ni-S合金电极性能的影响
袁铁锤,周科朝,李瑞迪
(中南大学 粉末冶金国家重点实验室,长沙 410083)
摘 要:以氧化镨、氧化铒为添加剂,在泡沫镍基体上电沉积制备Ni-S镀层电极。对镀层的表面形貌、镀层结构、电沉积行为及镀层的电化学性能进行研究。结果表明:电镀液中添加稀土氧化物后电沉积过程的阴极极化增强,沉积层晶粒细化,比表面积增大,因而电极的析氢过电位降低;其中,添加Pr2O3的Ni-S镀层在250 mA/cm2下碱性水电解时过电位降低达37 mV,且该镀层电极在碱性介质下具有较高的析氢活性和耐腐蚀性能,在100 h水电解实验中表现出较强的稳定性。
关键词:氧化镨;氧化铒;Ni-S合金;电沉积;析氢反应
中图分类号:TQ 153.2 文献标识码:A
Effect of additives Pr2O3 and Er2O3 on properties of
electrodeposited nickel sulphur alloy electrode
YUAN Tie-chui, ZHOU Ke-chao, LI Rui-di
(State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China)
Abstract: Ni-S coating electrodes were prepared on the foam nickel substrates by electrodeposition method with Pr2O3 or Er2O3 as additives. The effects of the rare earth compounds on the grain sizes, textures, microstructures and electrochemical activities of Ni-S coatings were studied. The results show that the rare earth oxides added in electrolyte can increase the cathode polarization. The Ni-S coatings with additives have larger surface area and smaller grain sizes. The hydrogen evolution overpotential of Ni-S(Pr2O3) alloy coatings decreases, it exhibits higher hydrogen evolution activities and better corrosion resistant performance in alkaline water. The hydrogen evolution overpotential of Ni-S(Pr2O3) coatings electrolyzed at 250 mA/cm2 is 37 mV lower than that of the Ni-S coatings without additives. Most importantly, Ni-S(Pr2O3) coating exhibits high electrochemical stability for 100 h electrolysis.
Key words: Pr2O3; Er2O3; Ni-S alloy; electrodeposition; hydrogen evolution reaction
氢能作为一种高效、洁净的二次能源受到广泛关注。其中碱性电解水制氢是当前最常用的一种制氢方法[1],而过高的析氢过电位使得电解时的槽电压大,能耗增加,为降低电解能耗,必须开发新型廉价的高析氢性能电极材料[2-4]。Pt和Pd等贵金属虽具有很好的析氢性能,但由于其价格昂贵,不适于工业上推广应用[5-7]。因此,人们一直致力于研究新型的具有低析氢过电位的电极材料。
近年来,已经采用多种物理、化学方法制备析氢电极材料,其中采用离子镀、离子溅射和离子注入等物理方法[8]可以得到均匀、牢固的表层结构,但是工艺复杂,价格昂贵,不易用于大规模工业化生产。而电化学法尤其是电沉积法[9-12],具有工艺简单、成本低,镀层均匀、厚度易控、镀层成分及材料选择范围广等优点,因而被广泛使用。采用电沉积法制备电极材料时,由于镀液中加入少量稀土元素可改善镀液的分散能力,提高电流效率,同时还可改善合金电极的耐腐蚀性,使得稀土在电沉积制取析氢电极材料中的应用日趋广泛[13-16]。但有关稀土氧化物在电沉积Ni-S合金中的作用研究尚未见报道。本文作者通过在Watt(瓦特)浴体系中添加稀土氧化物Pr2O3和Er2O3,研究稀土氧化物添加剂对Ni-S镀层的电沉积过程及其电化学性能的影响。
1 实验
1.1 Ni-S合金镀层电极的制备
电极制备:阳极为1.5 cm×1.5 cm的镍片,阴极为1 cm×1 cm的孔隙均匀的泡沫镍,镍片及泡沫镍的镍含量均大于99.5%。电镀液的基本组成为瓦特浴体系:硫酸镍60~160 g/L,硫脲10~120 g/L,硼酸10~60 g/L,氯化钠5~40 g/L,氧化镨(粒径小于74 μm)0.5 g/L,氧化铒(粒径小于74 μm) 0.5 g/L,添加剂和表面活性剂适量;电沉积采用双阳极单阴极,温度为45 ℃,电流密度为30 mA/cm2,pH值为4.0~4.3,沉积时间为 60 min,电沉积后的样品用去离子水清洗后自然干燥。
1.2 电极表面形貌和结构
用扫描电镜(JSM-6360LY)对镀层表面形貌进行观察,用面能谱(EDX-GENESIS 60S)分析镀层表面元素含量,用XRD(D/MAX-RB)分析镀层物相组成、晶粒尺寸和结晶状态。
1.3 电化学性能测试
用CHI660b(USA)电化学工作站测试电极的电化学性能。其中,待测电极为工作电极,工作电极一面涂敷AB胶,使待测电极的工作面积为1 cm2,参比电极为饱和甘汞电极,辅助电极为铂丝,用仪器欧姆补偿功能自动校正测试溶液的电位降。测试电沉积Ni-S合金过程的阴极极化曲线和微分电容时,用上述制备电极的电镀液作电解液;测试Ni-S合金镀层电极的阴极极化曲线时,用30% KOH水溶液作电解液。
2 结果与讨论
2.1 稀土氧化物对Ni-S电沉积过程的影响
图1所示为泡沫镍基体上电沉积Ni-S合金的阴极极化曲线。由图1可见,在Ni-S镀液中加入稀土氧化物后,电沉积过程的阴极极化略有增强,添加Pr2O3的阴极极化增强效果更加明显。这是由于细粒度稀土氧化物吸附在基体表面阻碍了Ni2+,Ni[CS(NH2)2]2+和Ni[CS(NH2)2]22+络合离子的电沉积和界面扩散,减缓了阴极电子和溶液中放电离子的交换速度,阻碍镀层的生长,使阴极极化增大,有利于晶核的形成,使得电沉积过程中的晶核形成速度增大,而晶粒生长速度变慢。因此,沉积层的晶粒变细,晶粒数增多,镀层的活性表面积得到提高,增加了电极活性,降低了析氢过电位。
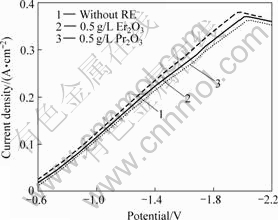
图1 泡沫镍基Ni-S合金电沉积过程的阴极极化曲线
Fig.1 Cathode polarization curves of Ni-S alloy during electrodepositing on foam nickel substrate
2.2 稀土氧化物对泡沫镍基Ni-S镀层电极析氢性能的影响
图2所示为3种镀层电极在30% KOH溶液中的阴极极化曲线。可以看出,与未添加稀土氧化物的电极相比,在相同电流密度下,Ni-S(Pr2O3)和Ni-S(Er2O3)电极的析氢过电位有所降低,其中Ni-S(Pr2O3)电极的过电位降低较多:在电流密度为250 mA/cm2下电解时,Ni-S(Pr2O3),Ni-S(Er2O3)镀层比Ni-S镀层析氢过电位分别降低约37 mV和10 mV。根据Tafel方程式计算得到3种电极的析氢反应动力学参数,见表1。由表1可以看出:Ni-S(Pr2O3)和Ni-S(Er2O3)电极的表观交换电流密度J0较Ni-S电极的大,这证明Pr2O3或Er2O3的加入可以提高电极材料的电催化活性。析氢催化活性除了与电极材料有关外,还与电极表面粗糙程度有关,电极表面粗糙度越大,越有利于氢的脱附。为此,用电位跃迁法测定了电极的微分电容值Cd,电位跃迁的幅度?φ为10 mV时,Cd值可视为一恒定值,即Cd=Q/?φ[15](Q为双电层充电时的总电荷量)。假设平滑金属表面的平均微分电容为20 ?F/cm2,则电极的比表面积Sreal = Cd/20,电极的粗糙因子r = Sreal/Sapp。测量后计算所得的数据如表2所示。由表2可以看出,添加稀土氧化物后表面粗糙度有所增加,这与析氢极化曲线的分析结果一致。
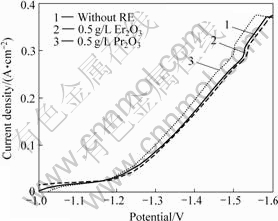
图2 泡沫镍基Ni-S镀层的阴极极化曲线
Fig.2 Cathode polarization curves of Ni-S coatings on foam nickel
表1 电极的析氢反应动力学参数
Table 1 Kinetic parameters of electrodes for HER
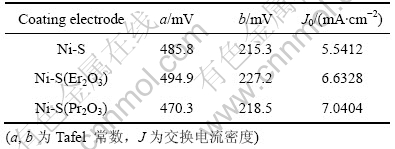
表2 电极的比表面积(Sreal)及相对粗糙因子(r)
Table 2 Real areas (Sreal) and roughness factors (r) of electrodes

耐蚀性也是电极材料的一项重要性能,耐蚀性强是保证电极材料使用寿命长、生产成本低的必要条件。图3所示为采用动电位扫描法测定的镀层在30% KOH溶液中的阳极极化曲线。由图3可以看出,Ni-S电极活化溶解电位较Ni-S(Pr2O3)和Ni-S(Er2O3)低约0.03 V,随着电位的正移,活化溶解电流逐渐增大,Ni-S和Ni-S(Er2O3)电极未显示出明显的钝化区,Ni-S(Pr2O3)电极在0.55~0.65 V区间有一明显钝化区。可见,镀液中加入稀土氧化物后镀层的阳极极化程度变大,即在相同的电极电位下,镀液中加入氧化镨、氧化铒后得到的镀层的溶解电流有所减小,镀层的耐腐蚀性能有所增强。
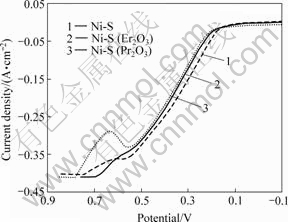
图3 镀层的阳极极化曲线
Fig.3 Anodic polarization curves of coatings
此外,电极的电化学稳定性是影响其工业化应用及使用寿命的关键因素。Ni-S(Pr2O3)电极具有较好的析氢性能,为研究该电极的稳定性,在60 ℃,30% KOH溶液中,进行100 h连续电解实验(电流密度J=250 mA/cm2),实验结果如图4所示。由图4可知,该电极在电解最初的10 h内,析氢电位由-1.495 V直线下降到-1.45 V,在接下来的10 h,析氢电位又由-1.45 V升高到-1.48 V,随后,析氢电位稳定在-1.49~-1.47 V之间,表现出较强的电化学稳定性。这是因为在电解过程中,起催化活性作用的是电极中的S,电解初期,随着部分覆盖在电极表面杂质的溶解,电极中S的催化作用显现出来,使得析氢电位降低较快;随着电解过程的进行,电极中的S出现溶解损失,S含量降低,析氢电位升高;一段时间后,镀层表面失S速度减慢,镀层中S含量变化小,残余的S及结合紧密的组织结构使镀层表现出较强的电化学稳定性,保证了电极长时间电解的稳定性。

图4 Ni-S(Pr2O3)电极析氢电位随时间的变化关系(250 mA/cm2)
Fig.4 Relationship between potential and time of Ni-S (Pr2O3) electrode under long-term electrolysis (250 mA/cm2)
2.3 Pr2O3对泡沫镍基Ni-S镀层形貌及结构的影响
图5所示为Ni-S镀层的表面形貌。由图5可知,未添加稀土的镀层表面较平坦,Ni-S(Pr2O3)镀层表面由于有微细的Pr2O3粒子吸附而明显变得粗糙。这是因为:一方面,当镀液中加入Pr2O3粒子后,电极表面某些活性点会吸附Pr2O3粒子,这样,该活性点附近的Ni2+及其络合离子的浓度降低,导致该处的晶粒生长被抑制而形成凹谷,未吸附Pr2O3粒子的区域镀层加速生长形成尖峰;另一方面,随着电沉积反应的进行,由于扩散传质的原因,凹谷处由于浓差极化被抑制生长,而尖峰处的电化学极化和浓差极化较低能够择优生长,进而形成粗糙表面。
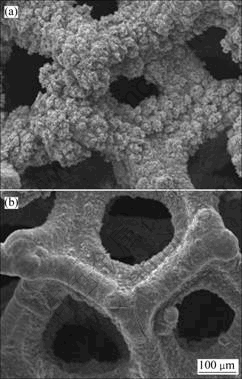
图5 泡沫镍基Ni-S镀层的SEM微观结构
Fig.5 SEM micrographs of nickel sulphur coatings on foam nickel substrates with (a) and without Pr2O3 (b)
对上述镀层的EDX能谱进行分析,结果表明,加入稀土氧化物Pr2O3后镀层的硫含量由19.94%下降到15.39%,这是由于Pr2O3在沉积界面的吸附阻碍了S2-的沉积,导致镀层中的硫含量有所降低。
Ni-S电极与Ni-S(Pr2O3)电极的XRD谱如图6所示。由图6可知,2种电极均有较强的镍衍射峰,但XRD图谱中并无S元素出现,而EDX能谱分析结果表明Ni-S电极中S含量为19.94%,Ni-S(Pr2O3)电极中S含量为15.39%,因此,可以认为Ni-S和Ni-S(Pr2O3)电极中Ni-S以非晶态合金的形式存在,而XRD谱中衍射峰显示的是镍基体峰。
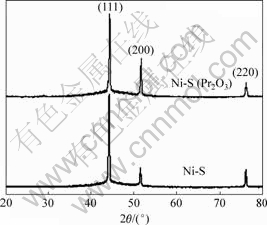
图6 镀层电极的XRD谱
Fig.6 XRD patterns of coating electrodes
3 结论
1) 在电沉积过程中,氧化镨、氧化铒均对Ni2+及络合离子电沉积起阻碍作用,从而减缓了阴极放电离子的交换速度,增大了阴极极化,促进了沉积层的晶粒细化,增大了沉积层的真实表面积和表面粗糙度。
2) 添加氧化镨、氧化铒均能提高Ni-S电极的表观电流密度和析氢催化活性。在电流密度250 mA/cm2下电解时,Ni-S(Pr2O3)和Ni-S(Er2O3)镀层电极比Ni-S镀层电极的析氢过电位分别降低约37 mV和10 mV。
3) 从添加了Pr2O3的Ni-S镀层电极的阳极极化曲线可见,镀层具有较小的溶解电流和较长的钝化区,表现出良好的耐腐蚀性能。镀层电极在经历最初10 h电解活化后,再经100 h连续电解,其析氢电位始终稳定在-1.49~-1.47 V之间,表明该电极具有较强的电化学稳定性。
REFERENCES
[1] 陈丹之. 氢能[M]. 西安: 西安交通大学出版社, 1990: 82.
CHEN Dang-zhi. Hydrogen energy[M]. Xi’an: Xi’an Jiaotong University Press, 1990: 82.
[2] HAN Q, LIU K, CHEN J. A study on the electrodeposited Ni-S alloys as hydrogen evolution reaction cathodes[J]. Inter J Hydrogen Energy, 2003, 28(11): 1207-1212.
[3] 熊建民, 丁云杰, 王 涛, 吕 元. La2O3助剂对Co/AC催化剂上费-托合成反应性能的影响[J]. 催化学报, 2005, 26(10): 874-878.
XIONG Jian-min, DING Yun-jie, WANG Tao, L? Yuan. Effect of La2O3 promoter on reaction performance of Fischer-Tropsch synthesis over Co/AC catalyst[J]. Chinese Journal of Catalysis, 2005, 26(10): 874-878.
[4] 吴玉琪, 吕功煊, 李树本. CoO改性TiO2光催化从水析氢光电化学行为研究[J]. 化学学报, 2005, 63(8): 671-676.
WU Yu-qi, L? Gong-xuan, LI Shu-ben. Photoelectrochemical performance of CoO-loaded TiO2 photocatalysts for hydrogen generation from water[J]. Acta Chimica Sinica, 2005, 63(8): 671-676.
[5] PASEKA I. Sorption of hydrogen and kinetics of hydrogen evolution on amorphous Ni-Sx electrodes[J]. Electrochimica Acta, 1993, 38(16): 2449-2454.
[6] YUAN Tie-chui, LI Rui-di, ZHOU Ke-chao. Electrolytic properties of Ni-S-Co coating electrode in alkaline medium[J]. Transactions of Nonferrous Metals Society of China, 2007, 17(4): 762-765.
[7] 周科朝, 袁铁锤, 李瑞迪. 泡沫镍基Ni-S-Co镀层电极在碱性介质中的电催化析氢性能[J]. 中南大学学报: 自然科学版, 2007, 38(2): 186-189
ZHOU Ke-chao, YUAN Tie-chui, LI Rui-di. Electrocatalytic properities of Ni-S-Co coatings electrodeposited on nickel foam substrate for hydrogen evolution in alkaline medium[J]. J Central South Unviersity: Science and Technology, 2007, 38(2): 186-189
[8] 李青莲, 曲永和. 提高析氢阴极电催化活性的方法及其进展[J]. 陕西化工, 1994, 4: 5-7.
LI Qing-lian, QU Yong-he. The processes and progress of improving catalytic activities of hydrogen evolution reaction[J]. Shanxi Chemical Industry, 1994, 4: 5-7.
[9] 吴 梅, 魏子栋, 沈培康. 泡沫镍载碳化钨催化剂上的析氢反应[J]. 催化学报, 2007, 28(4): 307-311.
WU Mei, WEI Zi-dong, SHEN Pei-kang. Hydrogen evolution reaction on foam Ni-supported WC electrocatalyst[J]. Chinese Journal of Catalysis, 2007, 28(4): 307-311.
[10] ELEZOVIC N R, JOVIC V D, KRSTAJIC N V. Kinetics of the hydrogen evolution reaction on Fe-Mo film deposited on mild steel support in alkaline solution[J]. Electrochimica Acta, 2005, 50: 5594-5601.
[11] HU W K, LEE J Y. Electrocatalytic properties of Ti2Ni/Ni-Mo composite electrodes for hydrogen evolution reaction[J]. Inter J Hydrogen Energy, 1998, 23: 253-259.
[12] RAJ I A. Nickel-based, binary-composite electrocatalysts for the cathode sin the energy-efficient industrial production of hydrogen from alkaline-water electrolytic cells[J]. J Applied Electrochemistry, 1993, 28: 4375-4384.
[13] 汪继红, 费锡明, 李 伟. 稀土在电沉积镍-钴合金中的作用[J]. 化学研究与应用, 2003, 15(4): 535-537.
WANG Ji-hong, FEI Xi-ming, LI Wei. Function of rare earth compounds electrodeposition of nickel-cobalt alloy[J]. Chemical Research and Application, 2003, 15(4): 535-537.
[14] 刘淑兰, 覃奇贤, 成旦红. 电沉积Ni-La合金上的阴极析氢行为[J]. 应用化学, 1995, 12(5): 115-116.
LIU Shu-lan, QIN Qi-xian, CHENG Dan-hong. Study of the hydrogen evolution reaction of cathode on electrodeposited Ni-La alloy[J]. Applied Chemistry, 1995, 12(5): 115-116.
[15] 肖友军. 含稀土La的镍基合金电镀及其析氢电催化行为研究[J]. 南方冶金学院学报, 2004, 25(1): 55-57.
XIAO You-jun. Electrocatalytic activity for hydrogen evolution reaction on electrodeposited nickel base alloys containing rare earth La[J]. Journal of Southern Institute of Metallurgy, 2004, 25(1): 55-57.
[16] HAN Qing, WEI Xu-jun, CHEN Jian-she. Properties of electrodeposited Ni-S(La) coatings[J]. Trans Nonferrous Met Soc China, 2002, 12(1): 54-56.
基金项目:国家高技术研究发展计划资助项目(2003AA305980)
收稿日期:2007-09-10;修订日期:2008-01-20
通讯作者:周科朝,教授,博士;电话:0731-8836418; E-mail: zhoukechao@mail.csu.edu.cn
(编辑 龙怀中)