锂离子电池碳负极中边缘碳及表面碳原子含量的理论计算
张 宝,郭华军,李新海,王志兴,彭文杰
(中南大学 冶金科学与工程学院,湖南 长沙,410083)
摘 要:通过理论分析与计算得到边缘碳及表面碳原子含量的表达式。分析了石墨微晶结构与成键特征,研究了石墨微晶中边缘碳原子与基平面碳原子的电化学特性。结果表明:边缘碳原子比基平面碳原子更易于与其他原子或基团形成较为稳固的联接,电化学反应活性较高;在首次充电过程中,边缘碳原子附近电解质的分解与SEI膜成膜反应速度较快,有利于形成联接较为紧密的SEI膜;建立了紧密堆砌的正六棱柱颗粒模型,推导出理想石墨中边缘碳原子及表面碳原子含量与微晶参数、颗粒尺寸之间的关系式。通过引入适当因子,修正了实际石墨颗粒与理想石墨在结构、形貌、孔隙率等方面的差别,得到的表达式可适用于石墨、无定形碳及改性碳等多种碳材料碳原子含量的计算。
关键词:锂离子电池;负极;碳;石墨
中图分类号:TM912.9 文献标识码:A 文章编号:1672-7207(2007)02-0251-05
Theoretical calculation of fraction of carbon atoms on surface and
edge for carbon anode of lithium ion battery
ZHANG Bao, GUO Hua-jun, LI Xin-hai, WANG Zhi-xing, PENG Wen-jie
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: The fractions of carbon atoms on the surface and edge were obtained by theoretical analysis and calculation. The electrochemical characteristics of carbon atoms on the edge and basal plane were determined by analyzing structure of graphite crystal and bond of different carbon atoms. The results show that the atoms on the edge are more active for electrochemical reactions and tend to form stable bond with other atoms and groups. In the initial charge, the electrolyte decomposition and formation of solid electrolyte interface (SEI) on the edge are faster, and tighter SEI is formed there. A hexagonal close-packed model for graphite particle was developed. The fraction of carbon atoms on the surface and edge was derived in expression of crystallographic parameters and particle size. The practical graphite particle and the ideal graphite have some difference in structure, appearance and micro-cavity, which may cause the fractions of surface carbon and edge carbon change, and some correctional factors have been introduced to revise the difference. The revised expression is suitable for calculation on fraction of carbon atoms of carbon materials such as graphite, disordered carbon and modified carbon.
Key words: lithium ion battery; anode; carbon; graphite
碳负极材料的研究是目前锂离子电池研究中最为活跃的领域之一,锂离子电池碳负极材料种类繁多,各种碳材料的嵌锂特性差别较大[1-5]。碳材料的电化学性能与其结构有很强的相关性[6-11],进一步对其结构给出精确的表征,并揭示这种相关性,对现有材料的改进和新材料的发现意义重大,但目前尚未见到这方面的文献报道。
本研究从碳材料的基本结构单元—— 碳六元环出发,根据石墨的结构特征,分析石墨微晶中不同位置碳原子的电化学活性,推导边缘碳原子及表面碳原子含量与石墨微晶或颗粒的结构与特性参数的关系式,以期构筑联系碳材料的结构、特性与电化学性能的桥梁,为后续探索多种碳负极材料及改性碳材料的嵌锂作用机理提供理论基础和依据。
1 石墨的结构及不同位置碳原子的电化学活性
碳位于元素周期表中的第二周期第四主族,碳原子的电子排布为1s22s22p2。在石墨中,碳原子的1个2s轨道与2个2p轨道(2px与2py)杂化形成3个等同的sp2杂化轨道,分别指向正三角形的3个角,另外一个未参加杂化的2pz轨道则与此三角形的平面垂直。当许多碳原子在同一平面内相互接近而结合时,它们易于采用杂化轨道,彼此间(平面方向)以3个共价键形成六角环形网状结构(如图1(a)和(b)所示)。许多平行堆积的大片网状体分子通过范德华力(分子键)结合起来,形成了石墨的晶体结构[12]。
石墨层与层之间的相对位置有2种排列形式,因而形成2种石墨晶体:一种是六方晶系石墨,相邻的两基面相互错开,上层基面的1个碳原子处于下层基面的六角形网格的中央,每隔1层,碳原子的位置相同,成为ABABAB……的三维空间有序排列(如图1(b)所示)[12-13];另一种是菱面体晶系石墨,层与层之间的结合呈ABCABC……重叠,菱面体晶系石墨实际上是一种有缺陷的石墨,在高温下可转化为六方晶系石墨。大多数的天然石墨与人造石墨为六方晶系石墨。
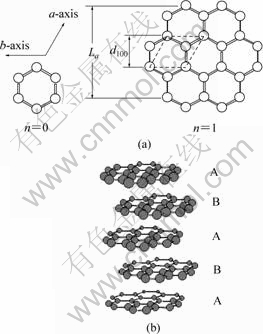
(a)carbon plane; (b)hexagonal crystallite
图1 石墨微晶结构示意图
Fig.1 Schematic diagram for structure of graphite crystallite
根据碳原子的结构示意图(图1),石墨微晶表面的碳原子可分为2类[14-15]:位于石墨微晶侧面的边缘碳原子,以及位于石墨微晶上下表面中的内层碳原子(本研究称之为基平面碳原子)。由于环境及成键情况的不同,基平面碳原子与边缘碳原子的电化学性质差别很大:
a. 边缘碳原子中有部分碳原子与相邻碳原子形成共价键的数目为2,在图1的模型中成键数为2的碳原子在边缘碳原子中的比例为(n+1)/(2n+1),这些碳原子的sp2杂化轨道上尚存在1个未成键电子,因而在合适的条件下易与周围的其它原子或基团如氧、氢、羟基、羧基等发生反应成键,而基平面内的碳原子由于sp2杂化轨道上的电子均已与相邻的碳原子形成共价键,它们只能以分子键的作用力与其他分子联接,即边缘碳原子与其他原子、分子、离子的作用力较强,联接较为稳固;
b. 边缘碳原子由于其成键的不饱和性,易于吸附其他原子、分子或离子,因此,在应用碳材料作电极时,其他原子、分子或离子易从边缘碳原子附近获得或失去电子而发生电化学反应,即边缘碳原子的电化学反应活性较高。
因此,位于石墨微晶表面的边缘碳原子具有比基平面碳原子高得多的电化学活性,在首次充电过程中,边缘碳原子附近的SEI膜成膜反应速度较快,而且有利于形成联接较为紧密的固体-电解质中间相(SEI)膜。
2 理想石墨颗粒中边缘碳原子及表面碳原子含量的计算
假设理想石墨碳层是由碳六元环按照图1方式均匀发展成正六边形,石墨微晶是由m层正六边形的碳层堆栈形成的正六棱柱,则有组成石墨微晶的碳层的二维尺寸[15]:

石墨微晶的堆栈厚度为

石墨微晶中每一碳层中的边缘碳原子数为
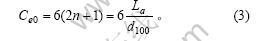
石墨微晶中m层碳层的边缘碳原子数为
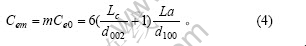
微晶中每一碳层中包含的碳原子数为
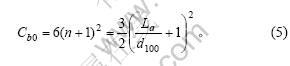
假设正六棱柱的石墨微晶紧密堆积形成正六棱柱的石墨颗粒。石墨颗粒底面的对角线长为D,高为H。则有石墨颗粒高度方向微晶层数为
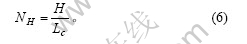
石墨颗粒中每一微晶层中包含的微晶数为石墨颗粒底面的面积与石墨微晶二维方向的面积之比:
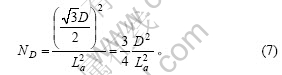
每一层石墨微晶层的六条边上包含的微晶数为
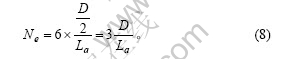
每一边缘微晶有2个侧面位于颗粒的外侧,微晶每侧的边缘碳原子数为Cem/6,则石墨颗粒中每一微晶层中边缘碳原子数为
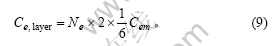
将式(4)及(8)代入式(9)并化简得
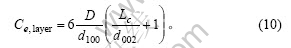
根据式(10)及式(6),石墨颗粒侧面边缘碳原子数为
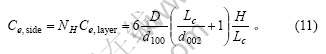
根据式(7)及式(9),颗粒上下2个底面中的碳原子数为
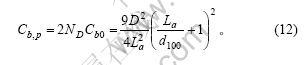
本文作者认为,上下两底面的碳层中,各微晶边缘的碳原子也是石墨颗粒中的一种边缘碳原子,根据式(3)及式(7),有
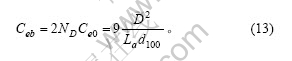
石墨颗粒中上下底面2个碳层中周边上的边缘碳原子既包括在式(11),又包括在式(12)和(13)中,由式(3)及(8)可计算出这部分碳原子数为
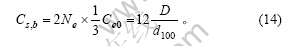
则石墨颗粒中的边缘碳原子数为
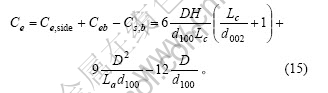
石墨颗粒中表面碳原子数为
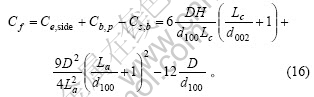
根据式(2)、(5)、(6)和(7)得石墨颗粒中碳原子的总数为
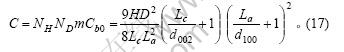
对于石墨材料,D?La,H?Lc,La?d100,Lc?d002,故式(15)和(16)中第3项(对应于Cs,b)与前2项相比可以忽略,这2式及式(17)可分别简化为

第2项对应于上、下底面微晶边界上的边缘碳原子。

石墨颗粒中总的碳原子数为:
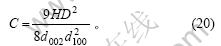
则边缘碳原子在石墨颗粒表面碳原子中的比例为
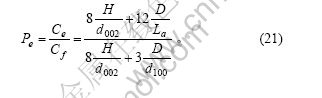
表面碳原子在石墨颗粒总的碳原子中所占的比例为
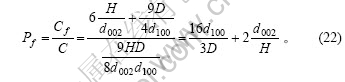
以上为理想石墨中的情形,在实际的石墨颗粒中,需对这些表达式进行一些修正。
3 实际石墨颗粒中边缘碳原子及表面碳原子含量的修正
在理想石墨的推导过程中,将石墨颗粒外形作为正六棱柱进行处理。事实上,石墨颗粒的底面可能为各种各样的多边形。在这里,先假设石墨颗粒外形为圆柱体或正四棱柱,且石墨颗粒的体积和高(H)不变,由此比较其形状变化对边缘碳原子和表面碳原子含量的影响。由于石墨颗粒的体积及高度不变,其底面积亦保持不变,上下底面的边缘碳原子及两底面中的碳原子数没有变化,则式(18),(19),(21)和(22)分别修正为
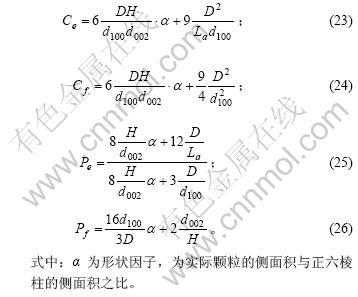
对于前面假设的圆柱体和正四棱柱形状的石墨颗粒,α分别约为0.952和1.075。可以认为,石墨颗粒的截面越接近于圆形,α越小,截面形状扁平、棱角突出或呈现了凹多边形时,α较大。
由于石墨微晶大小不一致及堆积程度不如理想状况下那么紧密,石墨颗粒中会产生许多微孔,其中有封闭式的,也有一端开口的,甚至存在通孔。但对石墨化度较高的碳材料来说,通孔的可能性较小。对于一端开口的微孔,只要孔径足够大,使得溶剂分子及溶剂化离子能够进入,其孔壁的碳原子作用与颗粒外表面的碳原子一样。同时,前面对石墨颗粒侧面形状的修正对微孔内壁同样有效,则式(23),(24)和(20)可修正为
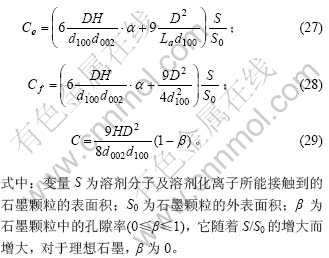
由于石墨颗粒中的边缘碳原子Ce与表面碳原子Cf以相同比例(S/S0)增加,因此,边缘碳原子在表面碳原子的比例Pe不变,与式(25)相同,而表面碳原子在总的碳原子中的比例Pf则变为
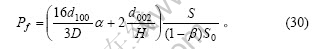
在前面的推导中,假设了组成石墨颗粒的各微晶的基平面均平行于上下底面。对于实际的石墨颗粒,由于石墨微晶之间的位错或取向不同,所有的石墨微晶的基平面并非平行于同一平面。因此,本文作者提出以下的几点假设:a. 一系列基平面相互平行的石墨微晶组成一个石墨微元(微小单元),石墨颗粒则是由许多取向不同的石墨微元构成,其中位于石墨表面(包括外表面和微孔内壁)的石墨微元有N个;b. 以正六棱柱来表征石墨微元(实际形状与正六棱柱之间的差别可以通过形状因子来修正),N个石墨微元的底面正六边形的对角线长及棱柱的高分别为Di和Hi;c. 石墨微元位于石墨表面的侧面面积占该石墨微元侧面积的比例分数为Ai,位于石墨表面的上下底面面积占该石墨微元上下底面积的比例分数为Bi(i=1,2,3,…,N),则石墨颗粒中的边缘碳原子及表面碳原子分别为这N个石墨微元的对应值之和。因此,式(23)和(24)变为
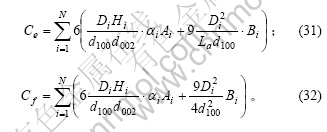
颗粒中总的碳原子数可表示为

根据Lc和La的定义及式(4),则有
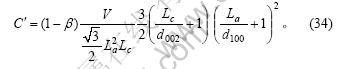
式中:Lc?d002,上式可简化为
 (37)
(37)
将式(31)~(37)与式(18)~(22)进行比较可知,在实际的情形中,D和H的物理意义分别为对应于紧密堆砌的正六棱柱石墨颗粒模型中的底面对角线及高度的有效值,且有
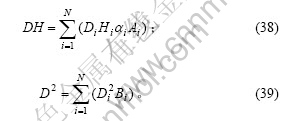
边缘碳原子及表面碳原子含量的表达式最初是由理想石墨的正六棱柱微晶模型及紧密堆积形成正六棱柱的石墨颗粒模型推导出来的,但是不难发现,经过一系列修正后,该表达式同样适用于石墨化程度较低的碳材料以及无定形碳材料中碳原子含量的计算,只是各表达式中修正系数的取值不同。
4 结 论
a. 石墨微晶中边缘碳原子易与其他原子或基团形成较为稳固的联接,电化学反应活性较高。
b. 建立了理想石墨的正六棱柱微晶模型及紧密堆砌的正六棱柱颗粒模型,推导出理想石墨中边缘碳原子及表面碳原子含量的计算公式。
c. 分析了实际石墨颗粒与理想石墨之间结构的差别,对计算公式进行了一系列的修正,并将其推广到其他碳材料碳原子含量的计算。
参考文献:
[1] Katsunori Y, Atsushi Y, Yoshinori K, et al. Carbon hybrids graphite-hard carbon and graphite-coke as negative electrode materials for lithium secondary batteries charge/discharge characteristics[J]. J Electrochem Soc, 2002, A149(7): A804-807.
[2] Guo Hua-jun, Li Xin-hai, Wang Zhi-xing, et al. Effect of lithium or aluminum substitution on the characteristics[J]. Rare Metals, 2003, 22(4): 280-284.
[3] Hongyu W, Masaki Y, Takeshi A, et al. Characterization of carbon-coated natural graphite as a lithium ion battery anode material[J]. J Electrochem Soc, 2002, A149(4): 499-503.
[4] Wu Y P, Rahm E, Holze R. Carbon anode materials for lithium ion batteries[J]. Journal of Power Sources, 2003, 114: 228-236.
[5] Guo Hua-jun, Li Xin-hai, Wang Zhi-xing, et al. Si-doped composite carbon as anode of lithium ion batteries[J]. Transactions of Nonferrous Metals Society of China, 2003, 13(5): 1062-1065.
[6] Michael E S, Henri W, Felix J, et al. Purely hexagonal graphite and the influence of surface modifications on its electrochemical lithium insertion properties[J]. J Electrochem Soc, 2002, A149(8): 960-966.
[7] Jung Y, Suh M C, Shim S C, et al. Lithium insertion into disordered carbons prepared from organic polymers[J]. J Electrochem Soc, 1998, A145(9): 3123-3129.
[8] Wang S, Yata S, Nagano J, et al. A new carbonaceous material with large capacity and high efficiency for rechargeable Li-ion batteries[J]. J Electrochem Soc, 2000, A147(7): 2498-2502.
[9] Shin R M, Takahiro H, Michiya T, et al. Reduction of irreversible capacities of amorphous carbon materials for lithium ion battery anodes by Li2CO3 addition[J]. Carbon, 2004, 42: 837-842
[10] GUO Hua-jun, LI Xin-hai, WANG Zhi-xing, et al. Mild oxidation treatment of graphite anode for Li-ion batteries[J]. J Cent South Univ Technol, 2005, 12(1): 50-54.
[11] Inagaki M. Textures in carbon materials[J]. New Carbon, 1999, 14(2): 1-13.
[12] 李圣华. 碳和石墨制品(上)[M]. 北京: 冶金工业出版社, 1983.
LI Sheng-hua. Products of Carbon and Graphite (I)[M]. Beijing: Press of Metallurgical Industry, 1983.
[13] 李士贤, 姚 建, 林定浩. 石墨[M]. 北京: 化学工业出版社, 1991
LI Shi-xian, YAO Jian, LIN Ding-hao. Graphite[M]. Beijing: Press of Chemical Industry, 1991
[14] Chung G C; Jun S H; Lee K Y, et al. Effect of surface structure on the irreversible capacity of various graphitic carbon electrodes[J]. J Electrochem Soc, 1999, A146(5): 1664-1671.
[15] Zaghib K, Nadeau G, Kinoshita K. Effect of graphite particle size on irreversible capacity loss[J]. J Electrochem Soc, 2000, A147(6): 2110-2115.
收稿日期:2006-06-12
基金项目:国家自然科学基金资助项目(50302016); 中国博士后基金资助项目(2005037698)
作者简介:张 宝(1971-),男,江苏盱眙人,博士后,从事材料及电化学研究
通讯作者:张 宝,男,博士后;电话:0731-8836357;E-mail: zhangb@qianlong.com