文章编号: 1004-0609(2006)03-0542-08
大洋多金属锰结核酸浸贵液中铁锰元素的脱除
孙春宝1, 吕继有1, 李浩然2
(1. 北京科技大学 土木与环境工程学院, 北京 100083;
2. 中国科学院 过程工程研究所, 北京 100080)
摘 要: 研究了大洋多金属锰结核酸浸贵液中铁锰元素与铜、 钴、 镍有价金属的分离。 根据溶液所含金属的特点, 选用可以回收利用的MnO2作为Fe2+的氧化剂先将Fe2+氧化为Fe3+; 采用黄铵铁矾法与Fe(OH)3相结合的二步法除铁工艺, 并对溶液pH、 反应温度、 反应时间等操作参数进行优化, 铁的沉淀率达到99.8%, 净化后溶液中含铁量低于0.01g/L, 钴、 铜、 镍、 锰的回收率分别达到99.5%、 93.3%、 99.6%、 99.3%; 硫化沉淀分离锰和铜、 钴、 镍过程中, 硫化钠适宜用量为理论用量的4.5倍, 适宜pH值4.5, 适宜温度80℃, 沉淀时间1h, 铜、 钴、 镍的沉淀率在99%左右, 而锰的沉淀率仅为0.46%。
关键词: 大洋多金属结核; 溶液净化; 沉淀法 中图分类号: TF111.3
文献标识码: A
Removal of iron and manganese element from
ocean multimetallic nodules acid leaching solution
SUN Chun-bao1, Lū Ji-you1, LI Hao-ran2
(1. School of Civil and Environment Engineering, University of Science and Technology Beijing,Beijing 100083, China;
2. Institute of Process Engineering, Chinese Academy of Sciences,Beijing 100080, China)
Abstract: The removal of iron and manganese element from the ocean multimetallic nodules acid leaching solution was discussed. For being recycled, MnO2 was choosed to oxygenate Fe2+ to Fe3+ firstly. The two steps method was used to remove iron element from the solution, which consists of jarosite and Fe(OH)3 method, and the operation parameters such as pH value, reacting temperature, reacting time were optimized. The sedimentation rate of iron can reach 99.8%, the content of iron in the purified solution is below 0.01g/L .The recoveries of Co, Cu, Ni, Mn are 99.5%, 93.3%, 99.6%, 99.3% respectively. It is shown that the usage of Na2S is 4.5 times of theory usage, the suitable reacting parameters are pH 4.5, temperature 80℃, and reacting time 1h. The sedimentation rates of Co, Cu and Ni are 99%, the sedimentation rate of Mn is only 0.46%.
Key words: ocean multimetallic nodules; solution purification; sedimentation
大洋多金属锰结核是铁锰氧化物在深海中的沉积物, 形状各异, 直径多在20~100mm之间, 以覆盖或浅埋的方式赋存于5000~6000m海底。 大洋多金属锰结核中有工业提取价值的主要元素有Cu、 Co、 Ni、 Mn等, 其中Ni、 Co、 Cu均以吸附或离子取代形式嵌布于铁、 锰氧化物晶格中, 基本上没有机械分选富集的可能, 要提取这些有价元素必须先破坏铁、 锰氧化物晶格。 由于二氧化锰在常温常压下不与酸、 碱反应, 使得处理过程复杂化[1-12]。
自20世纪60年代以来, 对大洋多金属结核的加工处理提出了几十种方案, 其中研究较深入, 并经过扩大试验检验的主要有以下几种方法: 还原焙烧氨浸法[3, 13]、 亚铜离子氨浸法[1, 3]、 高温高压硫酸浸出法[10, 12]、 氯化氢还原焙烧浸出法[10]、 熔炼—硫化—浸出法[3]、 微生物浸出法[10]、 其他方法[1, 4]。 还原焙烧氨浸出法和亚铜离子氨浸出法的优势在于不浸出铁、 锰, 选择性强, 试剂腐蚀小、 消耗少且易回收, 缺点是浸出回收率低, 特别是钴回收率低, 且难回收锰; 高温高压硫酸浸出法选择性强, 矿石无需干燥, 可直接浸出, 工艺可靠, 缺点是钴回收率低, 不能回收锰, 设备材质要求高, 投资大。 氯化氢还原焙烧工艺的金属浸出回收率高(特别是钴), 可回收锰、 钴、 镍、 铜4种金属, 但试剂消耗量大且腐蚀性强, 回收锰的工艺不但复杂, 而且能耗高。 熔炼—硫化—浸出法的优点是金属回收率高, 可回收锰, 流程试剂消耗少。 但不论采用什么手段处理大洋多金属结核, 在浸出过程中, 除有价组分被浸出外, 铁矿物中的铁也被浸出, 进入浸出溶液中的铁及贱金属会造成产品的严重污染, 因此必须将铁或贱金属从溶液中除去。
锌冶炼过程中的沉淀除铁问题, 在湿法冶金中最具代表性, 许多重要的除铁工艺, 都是从湿法炼锌发展来的。 黄钾铁矾法[14-16]、 针铁矿法和赤铁矿法[16]作为新的除铁方法, 较好地解决了锌湿法冶金中的固液分离问题。
黄钾铁矾法除铁就是使溶液中的Fe3+离子在较高温度、 常压和有碱金属或铵离子存在的条件下, 从弱酸性硫酸盐溶液中或有足够硫酸根存在下的氯化物溶液中缓慢形成晶体沉淀。 它易于沉降、 过滤、 洗涤, 非常稳定, 在水中溶解度很低, 其中钾矾溶解度最低。 黄钾铁矾法的缺点是渣量大, 硫酸消耗较多。
针铁矿是含水氧化铁的主要矿物之一, 通常称为α型—水氧化铁, 它的组成为α-Fe2O3·H2O或α-FeOOH, 与纤铁矿(γ-FeOOH )是同质多象变体。 针铁矿法的要点是使溶液中的三价铁离子浓度在沉淀过程中保持较低水平, 如1g/L左右。 实现这一目标有两条途径: 即还原-氧化法(V·M法)和部分水解法(E·Z法)[17]: 还原-氧化法(V·M法)是先将溶液中的铁还原为二价, 然后在三价铁水解的条件下将二价铁缓慢氧化成三价铁, 使铁以针铁矿沉淀。 该工艺生产效率较低, 动力消耗大, 酸平衡较难掌握, 酸、 碱耗量较大, 设备较为复杂。 部分水解法(E·Z法), 是澳大利亚电锌公司发展的一种新的针铁矿法, 该法的特点是将三价铁的溶液缓慢而均匀地加入具备水解条件的溶液中, 加入速度不高于三价铁水解的速度, 使铁以针铁矿沉淀。
本文作者在充分借鉴国内外先进工艺的基础上, 采用黄钾铁矾法净化除铁, 硫化沉淀法实现锰和铜、 钴、 镍的分离, 为大洋多金属结核酸浸贵液中的有价金属的综合回收打下了基础。
1 实验
1.1 原料
采用的大洋多金属结核为DY105-11西小区8#拖网所采样品。 锰结核经风干后粉碎、 浸出。 浸出贵液中有价成分的含量列于表1[5]。
表1 浸出贵液中有价成分的含量(g/L)
Table 1 Content of valuable element in leaching solution (g/L)

1.2 实验设备
实验所用仪器设备如图1所示。
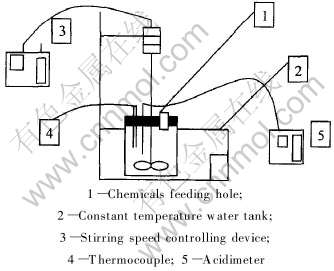
图1 净化实验装置示意图
Fig.1 Diagram of purifying equipment
1.3 实验流程
实验流程见图2。
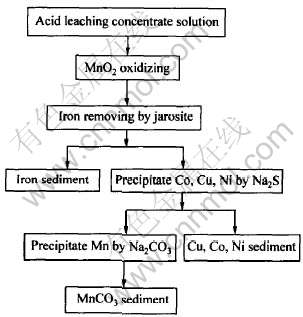
图2 实验流程图
Fig.2 Experimental flow chart
2 结果与分析
2.1 铁的去除
2.1.1 MnO2氧化实验
实验条件: 控制浸出液的pH为1.0~1.5, 温度95℃, 氧化反应1h。 实验结果列于表2。
由表2可以看出, 用MnO2作为Fe2+的氧化剂, 氧化前后溶液中的各金属含量(除锰外)没有明显变化。
2.1.2 pH值对黄铵铁矾沉铁的影响
黄铵铁矾法沉铁的反应机理如下:
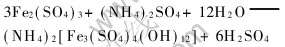
由黄铵铁矾的生成反应式可知, 溶液pH值对黄铵铁矾的生成和稳定性都有重要影响, 其影响规律列于表3。 当pH为1.0时, 黄铵铁矾的晶核生成较缓慢, 1h后还没有晶体析出; 另外, 在黄铵铁矾的生成过程中溶液pH值会下降。 由表3可知, 溶液最佳的初始pH范围为1.5~2.0。
2.1.3 反应温度对黄铵铁矾法沉铁效果的影响
在溶液初始pH值为2.0, 硫酸铵的用量为黄铵铁矾法理论消耗量, 反应时间4h的条件下, 温度对黄铵铁矾法除铁率及钴损失的影响结果如图3所示。 可以看出, 在黄铵铁矾法除铁的实验中, 反应温度对铁的沉淀影响很大, 当温度低于90℃时, 随着温度的增高, 铁的沉淀率增加, 但达不到合格要求。 说明温度低于90℃时, 黄铵铁矾的生成速度缓慢, 除铁率低, 而且沉淀的结晶不好, 过滤性能变差, 钴的损失也较多。 当温度大于90℃时, 铁的沉淀率较高, 且钴的损失率较低, 说明要获得好的除铁效果, 沉铁过程的温度必须控制在90℃以上。
表2 MnO2氧化实验结果
Table 2 Results of MnO2 oxidizing experiment
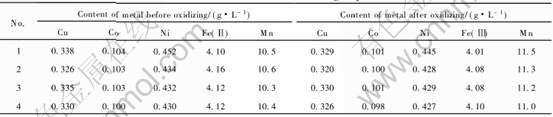
表3 pH值对黄铵铁矾沉铁的影响
Table 3 Effects of pH value on iron sediment

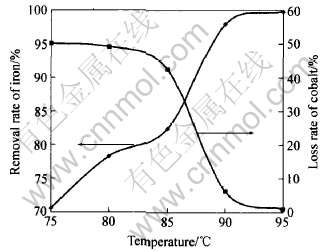
图3 温度对除铁率和钴损失率的影响
Fig.3 Effects of temperature on removal rate of iron and loss rate of cobalt
2.1.4 反应时间对沉铁的影响
在溶液初始pH值为2.0, 反应温度90℃, 硫酸铵的用量为黄铵铁矾法理论消耗量, 溶液终止pH值为4.5条件下, 分别进行反应时间为2.5、 3.0、 3.5、 4.0、 4.5h的沉铁实验, 实验结果如图4所示。 可以看出, 4h后反应基本上达到平衡, 说明黄铵铁矾法除铁的过程中, 黄铵铁矾晶体的生成需要一定的时间, 反应时间越长, 除铁效率越好。 综合考虑各方面因素, 沉铁的反应时间定为4h。
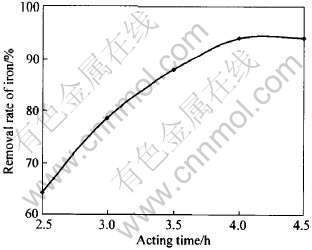
图4 反应时间对黄铵铁矾法沉铁效果的影响
Fig.4 Effect of reacting time on iron sedimentation
2.2 锰与铜、镍、钴的硫化沉淀分离
由于锰与铜、钴、镍的硫化物的溶度积有较大差别, 可选用Na2S作硫化剂, 采用硫化沉淀法分离锰和铜、 钴、 镍。
2.2.1 pH值对硫化沉淀的影响
取分离铁后的贵液, 控制Na2S加入量为理论用量的4.5倍, 在80℃下机械搅拌1h, Na2S溶液的浓度为20%, pH值变化对金属的沉淀率影响结果如图5和图6所示。 从图5可以看出, pH值控制在4.5较为适宜。 pH值在5.0虽然可获得更好的沉淀回收率, 但CoS、 NiS和CuS的纯度有所降低。 因为当pH值为5时, 锰的沉淀率上升, 当pH值达到6.0时, 锰的沉淀率为12.8%。

图5 pH值对铜、 钴、 镍沉淀率的影响
Fig.5 Effect of pH value on sedimentation rate of Cu, Co, Ni

图6 pH值对锰沉淀率的影响
Fig.6 Effect of pH value on sedimentation rate of Mn
2.2.2 Na2S用量对硫化沉淀效果的影响
取除铁后的贵液, 控制pH值4.5, 在80℃下机械搅拌1h, 调节Na2S溶液的浓度为20%, Na2S加入量对沉淀效果的影响如图7和图8所示。

图7 Na2S用量对铜、 镍、 钴沉淀率的影响
Fig.7 Effects of dosage of Na2S on sedimentation rate of Cu, Ni, Co

图8 Na2S用量对锰沉淀率的影响
Fig.8 Effect of dosage of Na2S on sedimentation rate of Mn
图7和图8表明, Na2S沉淀分离铜、 钴、 镍和锰, 其最适宜的用量为理论用量的4.5倍。 由于在Na2S加入的过程中, Na2S容易发生水解反应, 故其用量与加入方式、 加入速度都有一定的关系, 本实验采用滴加的方式。 在本实验中, 若Na2S用量为理论用量的5倍时, 铜、 钴、 镍的沉淀回收率都 有所增加, 但锰的沉淀率也增加, 故综合考虑, 选择Na2S用量为理论用量的4.5倍。
2.2.3 碳酸锰的制备
常规生产碳酸锰粉是用碳铵复分解硫酸锰, 以制取工业级碳酸锰。 在此用低浓度碳酸钠溶液对硫酸锰溶液进行碳化处理制得碳酸锰粉, 具体操作是用沸腾过的水配制质量浓度为3~5g/L的Na2CO3溶液, 在中速搅拌下向硫酸锰溶液中缓慢加入Na2CO3溶液; 当溶液pH达到6.8时, 碳化已完成, 此时应停止加入Na2CO3; 滤出碳酸锰, 并经3~4次清水(或去离子水)洗涤、 干燥后, 得到高纯碳酸锰粉。
2.3 酸浸贵液净化机理分析
2.3.1 溶液净化除铁机理
溶液中含有铜、 镍、 钴、 锰、 铁(二价和三价)等金属, 常用的除铁方法有黄铁矾法、 针铁矿法和Fe(OH)3水解法等。
不论哪种除铁方法都与溶液中金属氢氧化物的Ksp、 aMen+和pH值密切相关。 因此, 必须对溶液中各种金属离子的浓度、 金属氢氧化物的Ksp和溶液pH之间的关系进行分析研究, 以确定实验的适宜条件。
金属阳离子在溶液中生成难溶氢氧化物的反应可用下面通式表示:
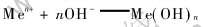
由上式可以得出氢氧化物沉淀时, pH、 aMen+和Ksp三者之间的关系:

当溶液中Fe、 Co、 Ni、 Mn的aMen+=1时, 二价和三价金属氢氧化物达到平衡时的pH0和Ksp的关系列于表4。
表4 25℃、 aMen+=1时, 铁、 钴、 锰氢氧化物的pH0和Ksp的关系
Table 4 Relationships between pH0 and Ksp of Fe, Co, Ni hydroxide under 25℃, aMen+=1

从表4可以看出, 二价铁稳定存在时的pH≤6.7, 二价钴稳定存在时的pH≤6.3, 因此, 对Fe2+和CO2+采用直接水解沉淀法不可能达到较好的分离。 但如果将Fe2+氧化成Fe3+, Fe3+稳定存在时的pH≤1.6, 便可采用直接水解沉淀法使钴铁分离。 所以采用直接水解法除铁, 必须先将二价铁氧化成三价铁。
Fe3+易水解生成Fe(OH)3沉淀, 但这种沉淀通常是无定形的胶状物, 很难过滤, 同时还会造成大量钴、 铜的共沉淀损失, 故工业上常采用黄铁矾法或针铁矿法除铁。
黄铁矾除铁法是一种常压除铁的方法, 即在较高的温度和有碱金属或铵离子存在的条件下, 从弱酸性硫酸盐溶液中缓慢地形成碱式硫酸钾(钠、 铵)等复盐沉淀物。 该沉淀物非常稳定, 溶解度很低, 易于沉降过滤和洗涤。 硫酸盐溶液中沉淀铁的总反应如下:

式中 X+可以是K+、 Na+、 NH4+、 H3O+。
黄铁矾法要求溶液中有足够的硫酸根离子和碱金属或铵离子, 根据浸出贵液的特点, 实验采用黄铵铁矾法除铁。 由于黄铁矾法除铁不彻底, 一般残铁浓度大于0.2g/L, 所以在接近实验终点时将pH值调高, 采用Fe(OH)3水解法除铁, 已达到理想的除铁效果。
2.3.2 氧化剂的选择依据
无论采用黄铁矾法、 针铁矿法还是水解法除铁, Fe2+都难以除去, 且从铁矾除铁的基本原理可知: 溶液中的铁离子必须是三价的, 因此需使用氧化剂将Fe2+氧化成Fe3+。 工业上常用的氧化剂有H2O2、 NaClO、 KMnO4、 KClO3、 Cl2、 MnO2、 O2等。 标准状态下, 氧化剂和Fe、 Co、 Ni的φ—pH关系如图9所示。 可以看出, φ(Fe3+/Fe2+)值较低, 而φ(Co3+/Co2+)值较高, 电位介于两者之间的氧化剂有H2O2、 KMnO4、 KClO3、 MnO2等, 因此本研究选择MnO2做为氧化剂。
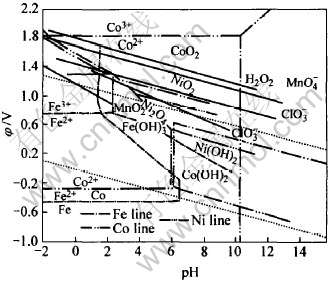
图9 标准状态下氧化剂和Fe、 Co、 Ni的φ—pH关系
Fig.9 φ—pH relation of oxidation and Fe, Co, Ni under standard state
2.3.3 硫化沉淀机理分析
硫化沉淀法是根据各种金属硫化物的溶度积不同, 在一定的条件下用硫化剂将金属离子进行分离。 常用的硫化剂有: H2S、 Na2S、 NaHS和Na2S2O3等。 本研究选用工业上常用的Na2S作硫化剂。
溶液中的S2-浓度与溶液pH值是密切相关的, 因此, 控制溶液的pH值就能达到控制溶液中的S2-浓度, 从而达到沉淀分离之目的。 水溶液中S2-浓度与pH值的关系如下:
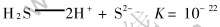
室温下, c(H2S)=0.1mol/L, 因此有
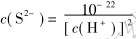
同理

则

由上式得到的金属离子硫化沉淀时c(S2-)、 pH和lgc(Men+)之间的关系如图10所示。
当金属离子浓度c(Men+)=0.01mol/L时, 金属硫化物沉淀时的pH值和相应的Ksp列于表5。
表5 难溶硫化物沉淀时的pH值和Ksp
Table 5 pH value and Ksp of sulphide precipitating (c(Men+)=0.01mol/L)
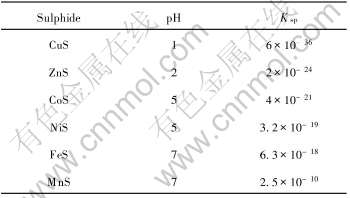

图10 金属离子硫化沉淀时c(S2+)、 pH值与lgc(Men+)的关系
Fig.10 Relations between c(S2+), pH value and lgc(Men+) while metal ion sulphide precipitating
从表5和图10可以看出, CoS、 CuS、 NiS和MnS的溶度积相差较大, 采用硫化沉淀法可以从含CO2-3、 Cu2+、 Ni2+、 Mn2+的溶液中沉淀钴、 镍、 铜而使钴、 镍、 铜和锰得到很好的分离。
采用硫化沉淀法沉淀时, pH值较低, 溶液为弱酸性, 溶液中有90%的Na2S按下式分解:

在实际反应过程中, 仅有部分Na2S直接与钴反应, 这就是研究过程中Na2S的用量超过理论用量很多的原因。
3 结论
1) MnO2氧化Fe2+的最佳工艺条件为: MnO2的用量为理论用量的1.5倍, pH值为1.0~1.5, 反应温度95℃, 氧化时间1h。
2) 黄铵铁矾与Fe(OH)3两步除铁的最佳工艺条件为: 初始pH值为2.0, 反应时间为4h, 反应温度90℃, 接近实验终点迅速提高pH值到4.5, 用50%的Na2CO3溶液热抽滤、 洗涤。 两步除铁法除铁率高且有价金属的损失小, 对设备要求较低, 反应容易控制。
3) 硫化沉淀分离锰和铜、 钴、 镍的适宜条件: pH值为4.5左右, 反应温度80℃, 反应时间1h, 硫化钠用量为理论用量的4.5倍, 铜、 钴、 镍的沉淀率在99%左右, 而锰的沉淀率仅为0.46%。 用低浓度碳酸钠溶液对硫酸锰溶液进行碳化处理制得碳酸锰粉。
4) 对大洋多金属结核酸浸贵液的脱铁、 脱锰机理进行了分析, 为大洋多金属结核酸浸贵液中有价金属的分离提纯和利用氢还原技术制取金属超细粉末提供了研究基础。
REFERENCES
[1]王淀佐, 张亚辉, 孙传尧. 大洋多金属结核的处理技术评述[J]. 国外金属矿选矿, 1996, 9: 3-11.
WANG Dian-zuo, ZHANG Ya-hui, SUN Chuan-yao, Evaluation of ocean multimetallic nodules processing technology[J]. Mineral Separation of Metal Overseas, 1996, 9: 3-11.
[2]张云, 管永诗, 田玉珍. 大洋锰结核资源的研究现状[J]. 矿产保护与利用, 2000(6): 39-42.
ZHANG Yun, GUAN Yong-shi, TIAN Yu-zhen. Current status of studies on the oceanic manganese nudole resources[J]. Conservation and Dutilization of Mineral Resources, 2000(6): 39-42.
[3]李新财. 日本大洋海底多金属结核加工方法研究的研究[J]. 国外金属矿选矿, 1998(2): 27-31.
LI Xin-cai. Research of processing methods of ocean multimetallic nodules in Japan[J]. Mineral Separation of Metal Overseas, 1998(2): 27-31.
[4]侯慧芬. 海洋锰结核的综合利用[J]. 上海有色金属, 1999, 20(3): 143-147.
HOU Hui-fen. Comprehensive utilization of ocean manganese nodules[J]. Shanghai Nonferrous Metal, 1999, 20(3): 143-147
[5]吕继有. 大洋多金属结核酸浸贵液的净化及氢还原制取超细金属粉末[D]. 北京: 北京科技大学, 2004.
L Ji-you. Purification of Ocean Multimetallic Nodules Acid Leaching Sodution and Making Thin Metal Powder by HydrogenDeoxidation[D]. Beijing: University of Science and Technology Beijing, 2004.
[6]张爱黎, 杨春芳. 铜厂副产品硫酸镍中除铁的研究[J]. 有色矿冶, 2001, 17(2): 23-2.
ZHANG Ai-li, YANG Chuen-fang. Study on removing iron element from byproduct of copper plant nickel sullfate[J]. Mining and Metallurgy of Nonferrous Metals, 2001, 17(2): 23-2.
[7]梅光贵, 钟竹前. 湿法冶金新工艺[M]. 长沙: 中南工业大学出版社, 1984. 184-193.
MEI Guang-gui, ZHONG Zhu-qian. New Technology of Hydrometallurgy[M]. Changsha: Central South University of Technology Press, 1984. 184-193.
[8]傅丽荣, 李嘉诚, 唐林生. 海洋锰结核酸性还原浸出的研究[J]. 青岛化工学院学报, 1996, 17(1): 30-33.
FU Li-rong, LI Jia-cheng, TANG Lin-sheng. Reducing leaching of sea manganese in the presence of sulfuric acid[J]. Journal of Qingdao Institute of Chemical Technology, 1996, 17(1): 30-33.
[9]江权. 锰的存在及应用[J]. 中国锰业, 2001, 19(8): 36-38.
JIANG Quan. Existance and application of manganese[J]. Managenese Industry of China, 2001, 19(8): 36-38.
[10]李浩然, 冯雅丽, 欧阳藩, 等. 微生物浸出深海多金属结核中有价金属的方法[P]. 中国专利00102747.6. 2000-10-27.
LI Hao-ran, FENG Ya-li, OUYANG Pan, et al. Microbe Leaching Valuable Element in Ocean Multimetallic Nodules[P]. CN 00102747.6. 2000-10-27.
[11]陈家镛, 杨守志, 柯家骏, 等. 湿法冶金的研究与发展[M]. 北京: 冶金工业出版社, 1998. 440-479.
CHEN Jia-yong, YANG Shou-zhi, KE Jia-jun, et al. Research and Development of Hydrometallurgy[M]. Beijing: Metallurgical Industry Press, 1998. 440-479.
[12]徐传华. 大洋多金属结核冶炼工艺技术经济评价[J]. 北京矿冶研究总院学报, 1994, 3(1): 55-61.
XU Chuan-hua. Technical and economic evaluation of smelting technology of ocean multimetallic nodules[J]. Journal of Bgrimm, 1994, 3(1): 55-61.
[13]侯慧芬. 海洋锰结核的综合利用[J]. 上海有色金属, 1999, 20(3): 143-147.
HOU Hui-fen. Comprehensive utilization of ocean manganeae nodules[J]. Shanghai Nonferrous Metals, 1999, 20(3): 143-147.
[14]董风书. 黄铁矾法和针铁矿法在有色冶金中的应用[J]. 重有色冶炼, 1979(3): 18-19.
DONG Feng-shu. Application of jarosite and rublinglimmer in nonferrous metal metallurgy[J]. Heavy-nonferrous MetaL Smelting, 1979(3): 18-19.
[15]Pammenter R V, Haigh C J. 采用低污染黄钠铁矾法提高金属回收率[J]. 有色冶炼,1983(5): 13-19.
Pammenter R V, Haigh C J. Increase metal recovery rate by low-polluting jarosite[J]. Nonferrous Metal Smelting, 1983(5): 13-19.
[16]陈家镛. 湿法冶金中铁的分离与利用[M]. 北京: 冶金工业出版社, 1991.
CHEN Jia-yong. Separation and Utilization of Iron Element during Hydrometallurgy[M]. Beijing: Metallurgical Industry Press, 1991.
[17]针铁矿法除铁技术资料[M]. 中南矿冶学院, 译. 长沙: 中南矿冶学院出版社, 1977.
Technical Matierial of Removing Iron Element by Rublinglimmer[M]. Central South College of Mining and Metallurgy, transl. Changsha: Central South College of Mining and Metallurgy Press, 1977.
[18]赤铁矿法处理锌浸出渣[J]. 杨建华, 译. 重有色冶炼, 1976(3): 36.
Zinc Leaching Slag Processing by Hematite[J]. YANG Jian-hua, transl. Heavy-nonferrous MetaL Smelting, 1976(3): 36.
基金项目: 中国大洋协会“十五”资源加工资助项目(Dy95-04-05)
收稿日期: 2005-06-13; 修订日期: 2005-12-01
作者简介: 孙春宝(1963-), 男, 副教授, 博士
通讯作者: 孙春宝, 副教授; 电话: 010-62333766; E-mail: suncb@ces.ustb.edu.cn
(编辑陈爱华)