文章编号:1004-0609(2017)-01-0128-10
N-异丁氧羰基硫脲浮选黄铜矿的机理
刘 微,刘广义,肖静晶,何芝玲,钟 宏
(中南大学 化学化工学院,长沙 410083)
摘 要:合成一种新型的表面活性剂——N-异丁氧羰基硫脲(iBCTU),对其结构进行红外和核磁共振表征,并考察其对黄铜矿的浮选性能以及在黄铜矿表面的吸附热力学和机理。浮选和吸附结果表明:在pH 3~11的范围内,iBCTU对黄铜矿具有良好捕收能力,且黄铜矿对iBCTU的吸附量随着温度的升高而增大。热力学分析表明:iBCTU在黄铜矿表面的吸附等温线符合Langmuir模型,吸附焓变ΔH为56.42 kJ/mol,熵变ΔS为284.76 J/(mol·K),吸附自由能变ΔG为-29.12 kJ/mol(298K),iBCTU在黄铜矿表面的吸附为自发、吸热化学吸附过程。XPS分析进一步说明iBCTU可能通过其硫代羰基的硫原子和—NH2基氮原子与黄铜矿表面的铜原子结合生成iBCTU-Cu(Ⅰ)表面络合物吸附在黄铜矿表面。
关键词:N-异丁氧羰基硫脲;黄铜矿;吸附热力学;浮选;XPS
中图分类号:TQ 311;O64 文献标志码:A
硫脲及其衍生物是重要的化工原料和有机化工中间体,具有通式为R—NH—C(=S)—NH—R'的一类化合物。硫脲及其衍生物普遍具有生物活性[1]和金属螯合性能 [2],应用广泛。在农业方面[3],硫脲类化合物可以用作杀虫剂、除草剂、植物生长调节剂和硝化抑制剂。在湿法冶金和矿物加工领域[4-9],硫脲类化合物对铜、银、金等金属离子具有优良的螯合作用,常用来分离和富集过渡金属。在医药领域[10-11],硫脲类化合物具有抗病毒,抗癌,抗疟疾和抗麻风等生物和药理活性。硫脲类化合物也可以用作金属缓蚀剂[12]。
N-烃氧羰基-N'-烃基硫脲是一类重要的硫脲表面活性剂,为铜矿物和贵金属矿物的高效捕收剂,能在弱碱性或中性 pH 矿浆中实现对硫化铜矿物的强力捕收与浮选回收[6-7, 9, 13-15]。NAGARAJ等[13, 15]认为N-烷氧羰基-N'-烷基硫脲是一类硫化铜矿浮选高效捕收剂,其分子中的硫原子和氧原子能够与铜、银等金属离子键合成六元环化合物。刘广义等[7, 9]发现在中碱度条件下N-乙氧羰基-N'-丙基硫脲捕收剂可高效实现硫化铜矿物与硫化铁矿物的浮选分离,显著降低铜/铁硫化矿物浮选分离的石灰用量,显著提高铜斑岩矿中铜矿物的浮选回收率。他们也采用X射线光电子能谱(XPS)等检测手段研究发现:N-异丙氧丙基-N'-乙氧羰基硫脲[16]和N-丁氧丙基-N'-乙氧羰基硫脲[17]通过它们分子中的硫代羰基硫原子和羰基与硫代羰基间的氧原子与黄铜矿表面的铜原子结合而生成四元环表面络合物。
N-烃氧羰基-N'-烃基硫脲(ROC(=O)NH—C(=S)—NH—R')表面活性剂是铜矿物的优良捕收剂,其中N'-烃基(—NH—R')可增加硫脲分子的疏水性,但当N-烃氧羰基(ROC(=O)NH—)中的烃基碳原子数足够多时,N'-烃基对硫脲分子疏水性的贡献率变小。为此,本文作者引入一类不含N'-烃基的新型N-烃氧羰基硫脲(ROC(=O)NH—C(=S)—NH2)表面活性剂,其用H原子取代N'-烃基。一方面,H原子取代N'-烃基有利于消除烃基R'对—NH—中氮原子的空间位阻,使硫脲分子中的活性位点更多,有望改善N-烃氧羰基硫脲表面活性剂在矿物表面的吸附,从而改善矿物颗粒表面疏水性,促进矿物浮选富集;另一方面,硫脲分子的表面活性可通过适当增加N-烃氧羰基中的烃基碳原子数来调节。
本文作者合成出一种新颖的黄铜矿浮选捕收剂—N-异丁氧羰基硫脲(iBCTU) CH3CH(CH3)CH2OC(=O) NH—C(=S)—NN2),并对其结构进行表征。研究iBCTU对黄铜矿的浮选性能和在黄铜矿表面的吸附行为。同时,通过吸附热力学和XPS分析了iBCTU在黄铜矿表面的吸附机理。
1 实验
1.1 试剂与仪器
N-异丁氧羰基硫脲根据相转移催化法制备[18]。其余化学试剂均购自商业公司,其纯度为化学纯及以上。黄铜矿源自墨西哥,购自加拿大Boreal科学公司,为手捡分选样品。试验、分析仪器主要有:SHA-C型水浴恒温振荡器(常州澳华仪器有限公司生产,中国),UV-1750型紫外可见分光光度计(岛津公司生产,日本),pHS-3C型pH计(上海精密科学仪器有限公司镭磁仪器厂生产,中国),Nicolet FTIR-740 型傅里叶变换红外光谱仪(尼高力公司生产,美国);AVANCE III500M型核磁共振波谱仪(布鲁克公司生产,瑞士);实验用水为一次蒸馏水。
1.2 矿样准备
黄铜矿经过手碎、手选和玛瑙研钵研磨,取38~76 um部分用于浮选试验,<38 um部分用于吸附试验(比表面积为0.783 m2/g(BET法))。黄铜矿矿样的XRF(X-ray fluorescence)分析结果见表1。实验前用超声波洗涤黄铜矿矿样,每次洗涤1 min,静置1 min后倒掉上层清液,重复洗涤3次,抽滤,自然晾干。
表1 黄铜矿XRF分析结果
Table 1 Element content of chalcopyrite by XRF (mass fraction, %)

1.3 iBCTU溶液标准曲线
准确称量0.176 g iBCTU,溶解在1000 mL蒸馏水中得到0.001 mol/L iBCTU溶液,稀释成各个浓度的溶液,在最大吸收波长256 nm处,用紫外分光光度计测定其吸光度(A),然后以吸光度为纵坐标,浓度为横坐标,得线性回归方程:A=0.00653+15866X,R2=0.9999。
1.4 吸附试验
移取50 mL一定初始浓度的iBCTU溶液于100 mL锥形瓶中,加入0.5 g黄铜矿,根据需要调节溶液pH或温度,置于恒温水浴振荡器中,转速为200 r/min,不同温度(298 K,303 K,308 K)下震荡一段时间,过滤,取上层清液测定其吸光度。
根据所得数据按照式(1)计算出任意时刻吸附量Qt。
 (1)
(1)
式中:c0为iBCTU溶液初始浓度,mol/L;ct为任意时刻iBCTU溶液浓度,mol/L;V为溶液体积,L;m为黄铜矿的加入量,g;Qt为任意时刻黄铜矿的吸附量,mol/m2;S为黄铜矿的比表面积,m2/g。
1.5 单矿物浮选实验
每次取已处理好的单矿物2.2 g于烧杯中,并向其中加入已调好浓度的220 mL iBCTU 溶液,搅拌1 min。用HCl或KOH调节矿浆pH值,再搅拌3 min后,加入1 mL浓度为3.3 g/L的甲基异丁基甲醇(MIBC)使其在浮选时浓度为15 mg/L,继续搅拌1 min后,静置30 s将上清液加入单泡管中,通入N2,流速控制在200 mL/min,加入矿,浮选3 min,收集泡沫产品和槽内产品,经干燥、烘干、称量质量后计算回收率。浮选回收率ε按式(2)计算。
 (2)
(2)
式中:m1为泡沫产品质量;m2为槽内产品质量。
1.6 XPS
采用ESCALAB 250Xi型X射线光电子能谱仪对iBCTU与Cu2+离子的反应沉淀以及黄铜矿表面吸附iBCTU前后进行检测,X射线源为Al Kα微聚集单色源,功率200 W,通能为20 eV,步长为0.1 eV,分析室真空度为1.33×10-6 Pa,仪器分辨率为0.45 eV,测量误差为0.2 eV,在通能为20 eV下得到S 2p、Cu 2p、N 1s、O1s及C1s的高分辨率光电子能谱。通过Thermo Avantage软件对高分辨率光电子能谱进行非线性拟合得到峰相关参数,实验得到的元素结合能以284.6 eV的C 1s进行校准。将打磨抛光后的黄铜矿用超声波清洗3次,浸泡在浓度为0.001 mol/L的iBCTU溶液中24 h,然后取出,蒸馏水洗涤,氮气吹干表面后置于玻璃真空干燥器干燥,用于XPS检测。将0.001 mol/L的iBCTU溶液500 mL与0.001 mol/L的铜离子溶液1000 mL混合,得到iBCTU-Cu沉淀,蒸馏水洗涤5次,置于真空干燥器干燥,用于XPS检测。
2 结果与讨论
2.1 iBCTU的合成与表征
由图1所示,将0.21 mol NaSCN和0.012 mol N,N-二甲基苯胺(DMA)加到含40mL水的三口瓶中,然后将混合体系冰浴冷却至0~15 ℃,滴加0.2 mol氯甲酸异丁酯,滴加完后,将反应混合物在0~15 ℃温度内反应4 h,得异丁氧羰基异硫氰酸酯中间体。然后在10 ℃以下滴加0.21 mol的氨水溶液,滴加完后反应1 h,过滤,粗产品用水和乙醇进行重结晶,得到iBCTU白色针状晶体,产率93%,熔程为147~148 ℃。
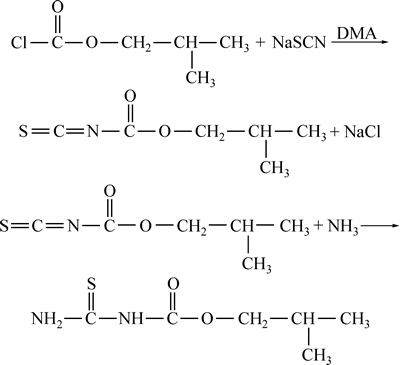
图1 iBCTU的合成路线
Fig. 1 Synthetic route of iBCTU
2.2 iBCTU对黄铜矿的浮选性能
在iBCTU浓度为5×10-5 mol/L,矿浆pH值对黄铜矿浮选回收率的影响如图2所示。由图2可知,在pH 3~11的范围内,iBCTU对黄铜矿具有良好捕收能力,黄铜矿的回收率均超过90%,表明iBCTU能在宽pH范围内浮选回收黄铜矿。
在矿浆pH值为8左右,iBCTU初始浓度对黄铜矿浮选回收率的影响见图3。图3表明,随着iBCTU浓度的增加,黄铜矿浮选回收率增加。当浓度增加到3×10-5 mol/L时,黄铜矿浮选回收率达到95.1%,之后随着药剂浓度的增加,回收率增长缓慢。
2.3 吸附试验结果
在iBCTU初始浓度为1.5×10-4 mol/L,不同温度(298K,303K,308K)下,黄铜矿对iBCTU的吸附量随时间的变化的关系曲线见图4。图4中结果表明,在同一温度下,吸附量随着时间的增加而增大,在吸附开始的0~20 h内,黄铜矿对iBCTU的吸附速率大,吸附量增长较快。超过20 h之后,随着时间的增加,吸附量增加变缓,在26 h基本达到平衡,同一吸附时间下,温度越高,吸附量越大,说明此过程是吸热过程,升高温度对吸附有促进作用。
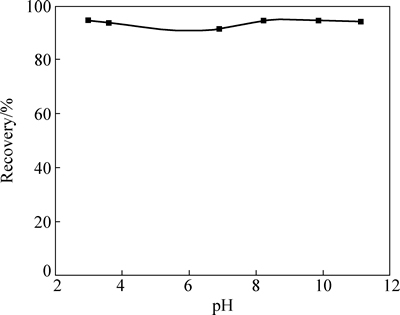
图2 pH和浮选回收率的关系
Fig. 2 Effect of pH values on flotation recovery of chalcopyrite
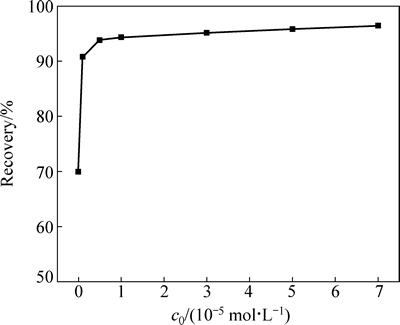
图3 iBCTU初始浓度和黄铜矿浮选回收率的关系
Fig. 3 Relationship between iBCTU initial concentration and flotation recovery rate of chalcopyrite
2.4 等温吸附
在自然pH=5.6下,将不同初始浓度的iBCTU溶液处理26 h,不同温度(298 K,303 K,308 K)下黄铜矿对iBCTU的平衡吸附量(Qe)与平衡浓度(ce)的关系见图5。
由图5可知,相同温度下,平衡浓度越大,黄铜矿对iBCTU的平衡吸附量也越大;在相同平衡浓度下,随着温度的升高,黄铜矿对iBCTU的平衡吸附量升高,说明该过程是吸热过程。
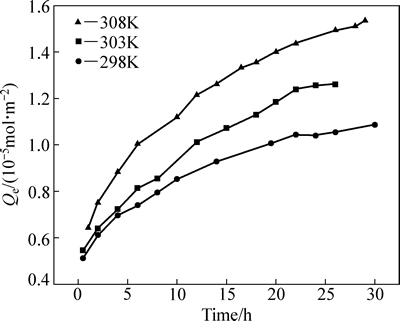
图4 黄铜矿对iBCTU的吸附量随时间变化的关系曲线
Fig. 4 Adsorption of iBCTU on chalcopyrite surface as a function of treatment time
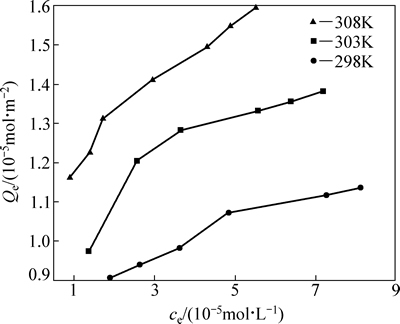
图5 黄铜矿对iBCTU的吸附等温线
Fig. 5 Adsorption isotherm of iBCTU on chalcopyrite surface
对等温吸附数据进行拟合,分别采用Langmuir[19](3)、Freundlich[20](4)等温吸附方程对图5中实验数据进行线性拟合,并对两者进行了比较,结果见图6和表2及图7和表3。
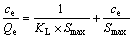 (3)
(3)
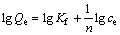 (4)
(4)
式中:KL为Langmuir常数;Smax为单位元最大吸附容量,mol/m2;Kf和n为Freundlich参数。
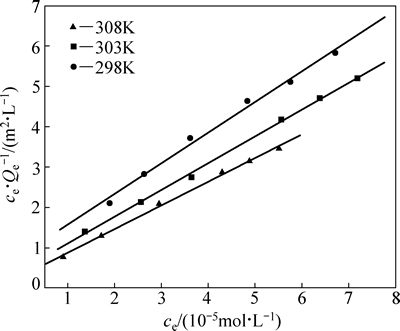
图6 图5中数据的Langmuir拟合曲线
Fig. 6 Langmuir fitting curves of data in Fig. 5
图6和表2以及图7和表3的拟合结果表明,采用Langmuir模型拟合所得的线性相关系数R2>0.99,比Freundlich等温方程拟合的更高,表明黄铜矿对iBCTU的吸附更符合Langmuir模型,说明该吸附过程可能是单分子层的化学吸附过程,可以进一步根据范特霍夫方程(5)和(6)来计算吸附热力学参数。
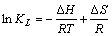 (5)
(5)
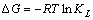 (6)
(6)
式中:KL 是吸附反应平衡常数;R是热力学气体常数,8.314 J/(mol·K);T为绝对温度;ΔH是吸附反应焓变,kJ/mol;ΔS是吸附反应熵变,J/(mol·K);ΔG吸附自由能变,kJ/mol。
ΔH和ΔS可通过lnKL对1/T作图所得斜率和截距求得,结果见图8和表4。
由图8和表4可以计算出在各个温度下ΔG<0,可知该吸附过程是自发进行的;ΔH=56.42 kJ/mol,焓变为正值,说明该吸附过程为吸热过程,与等温吸附结果一致。ΔS=284.76 J/(mol·K),ΔS为正值表明吸附过程为熵驱动过程而非焓驱动过程[21]。从微观上讲,对固液反应,根据吸附交换理论,iBCTU从水溶液迁移到固液界面并发生化学吸附,有较强的成键作用,iBCTU由无序趋向有序,此过程通常放热且熵减小。
表2 Langmuir等温方程拟合结果
Table 2 Fitting results of Langmuir isotherm equation
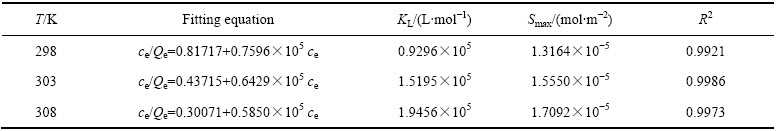
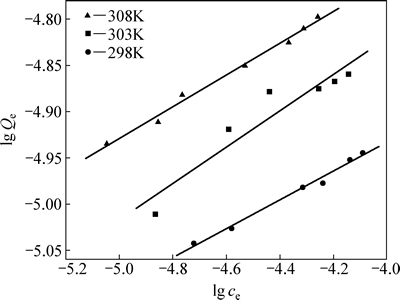
图7 图5中数据的Freundlich拟合曲线
Fig. 7 Freundlich fitting curves of data in Fig. 5
表3 Freundlich等温方程拟合结果
Table 3 Fitting results of Freundlich isotherm equation
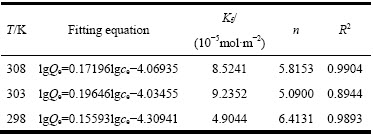
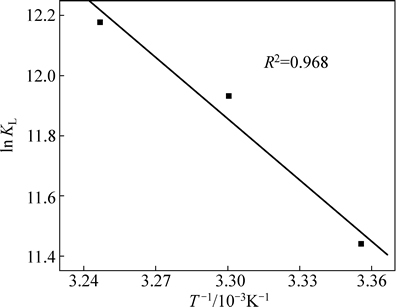
图8 lnKL/T -1关系图
Fig. 8 Relationship between lnKL and T -1
表4 在黄铜矿表面吸附的热力学参数
Table 4 Thermodynamics parameters of iBCTU adsorption on chalcopyrite surface

然而,黄铜矿表面吸附的水分子同时也会解吸到液相中,该过程需消耗能量且熵增大;并且,iBCTU分子含C=O,—NH—及—NH2官能团,在溶液中易与水分子形成氢键,其化学吸附于黄铜矿表面后必将削弱与水分子间的作用。因而,iBCTU在黄铜矿表面的吸附过程所消耗的能量可能大于释放的能量,熵增大于熵减,焓和熵都增加[22-23]。
2.5 XPS结果分析
iBCTU与黄铜矿作用前后及其与Cu2+反应产物iBCTU-Cu沉淀的XPS全谱见图9,各摩尔分数见表5。
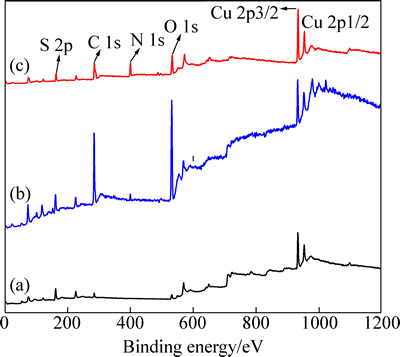
图9 黄铜矿吸附iBCTU前后及iBCTU-Cu沉淀的XPS谱
Fig. 9 Survey scan XPS of chalcopyrite before (a) and after (b) iBCTU treatment, and iBCTU-Cu precipitate (c)
表5 XPS测定的黄铜矿表面和iBCTU-Cu沉淀摩尔分数
Table 5 Mole fraction of elements for iBCTU-Cu precipitate and chalcopyrite surfaces as determined by XPS
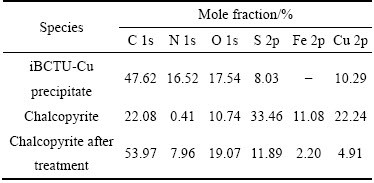
由图9和表5可知,iBCTU与铜离子形成的沉淀物iBCTU-Cu中的主要成分是Cu、N、C、O和S元素,且Cu、N、C、O的原子浓度与S的比值分别为1.28,2.06,5.93,2.18,表明iBCTU-Cu沉淀的化学组成大致为C6O2N2SCu(未考虑氢元素)。因此,iBCTU可能与铜离子按摩尔比1:1反应,生成iBCTU-Cu沉淀。黄铜矿经过iBCTU处理后,C、N、O元素的摩尔浓度均增加,S、Fe与Cu元素的原子浓度减少,表明iBCTU吸附在黄铜矿表面,降低了黄铜矿自身表面元素浓度。
iBCTU与黄铜矿作用前后及其与Cu2+反应产物iBCTU-Cu沉淀的元素XPS精细谱见图10~12,相应的XPS峰参数及化学态见表6~8。
iBCTU与黄铜矿作用前后及其与Cu2+反应沉淀iBCTU-Cu的Cu 2p3/2 XPS峰见图10,峰参数及化学态见表6。图10和表6的结果表明,黄铜矿中Cu 2p3/2 XPS 光谱出现在932.38 eV和934.45 eV处,分别归属于Cu(Ⅰ)和Cu(Ⅱ),其中Cu(Ⅱ)为黄铜矿表面铜的氧化物种[24],占9.09%。说明黄铜矿表面主要是硫化态Cu(Ⅰ)形式,少部分已被氧化为铜的氧化物或氢氧化物。iBCTU处理后,黄铜矿表面Cu 2p3/2 XPS在934.45 eV处的峰消失,而在932.68 eV处出现新的峰,这与iBCTU与铜离子反应产物iBCTU-Cu沉淀中932.53 eV处的峰相吻合,说明iBCTU在黄铜矿表面的吸附可将铜氧物中的二价铜还原成亚铜[25],同时以iBCTU-Cu表面络合物吸附在黄铜矿表面。
iBCTU与黄铜矿作用前后及其与Cu2+反应沉淀iBCTU-Cu的S 2p XPS峰见图11,峰参数及化学态见表7。图11和表7的结果表明,S 2p XPS谱比Cu 2p XPS谱复杂,每一个组成由两个高斯-洛伦兹峰所组成,其中S 2p3/2低能峰强度为S 2p1/2高能峰的两倍,它们的能量差约为1.18 eV。黄铜矿中S 2p3/2 XPS带出现在161.50 eV和163.08 eV处,分别归属于黄铜矿体相硫(S2-)[26]和表面聚合物硫(Sn2-、S2O32-)[27],这两种硫的含量比分别为76.14%和23.86%,说明黄铜矿表面部分硫已氧化。经iBCTU作用后,S 2p3/2XPS谱出现在161.17 eV和162.60 eV处,其中161.17 eV处归属于黄铜矿体相硫(S2-)[26],其含量为62.50%。体相硫(S2-)含量的降低进一步说明,由于iBCTU吸附在黄铜矿表面,使XPS能检测到的黄铜矿体相深度降低(XPS的检测深度为10 nm左右),体相硫检测量减少。S 2p3/2XPS谱在162.60 eV的硫可能归属于iBCTU-Cu表面络合物中的有机硫[28],其与iBCTU-Cu沉淀中S 2p3/2谱的峰值162.91 eV大致吻合。
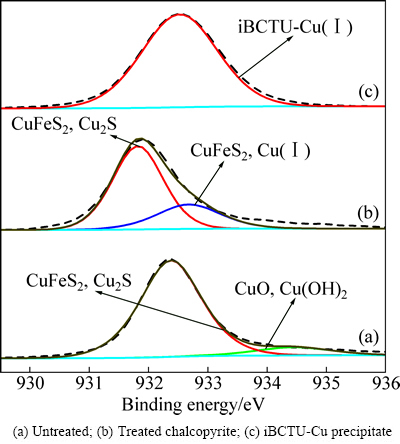
图10 黄铜矿吸附iBCTU前后及iBCTU-Cu沉淀的Cu 2p XPS精细图谱
Fig. 10 High-resolution Cu 2p XPS spectra
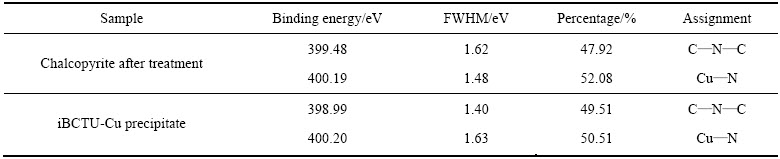
iBCTU与黄铜矿作用前后及其与Cu2+反应沉淀iBCTU-Cu的N 1s XPS峰见图12,峰参数及化学态见表8。图12与表8的结果表明,经iBCTU处理后,黄铜矿表面检测到两种状态的N原子,分别为结合能在399 eV附近的C—N—C[28-29]和400.2 eV处的Cu—N,摩尔比大致为1:1,这与iBCTU-Cu沉淀中N 1s XPS峰大致吻合。说明iBCTU((CH3)2CHCH2—O—C(=O)—NH—C(=S)—NH2)可能通过其氨基(—NH2)氮原子与铜原子成键,N原子中的电子向铜原子转移,使自身电子云密度降低,其N 1s XPS结合能升高。
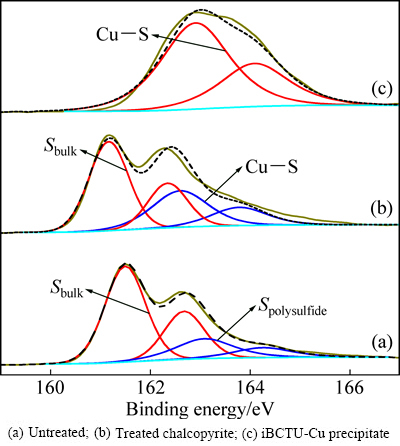
图11 黄铜矿吸附iBCTU前后及iBCTU-Cu沉淀的S 2p XPS精细图谱
Fig. 11 High-resolution S 2p XPS spectra
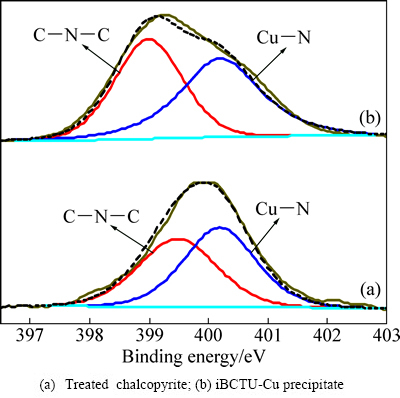
图12 黄铜矿吸附iBCTU后及iBCTU-Cu沉淀的N 1s XPS精细图谱
Fig. 12 High-resolution N 1s XPS spectra
表6 黄铜矿吸附iBCTU前后及iBCTU-Cu沉淀的Cu 2p3/2 XPS峰参数及化学态
Table 6 Deconvolution with Gaussian-Lorentzian bands of Cu 2p3/2 XPS spectra for untreated and treated chalcopyrite, and iBCTU-Cu precipitate
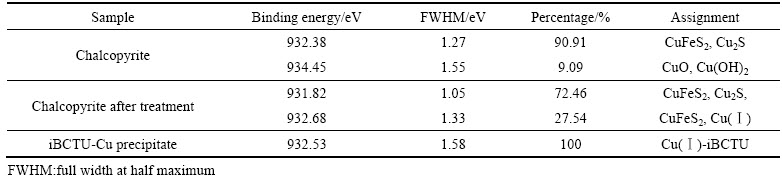
表7 黄铜矿吸附iBCTU前后及iBCTU-Cu沉淀的S 2p XPS峰参数及化学态
Table 7 Deconvolution with Gaussian-Lorentzian bands of S 2p XPS spectra for untreated and treated chalcopyrite, and iBCTU-Cu precipitate
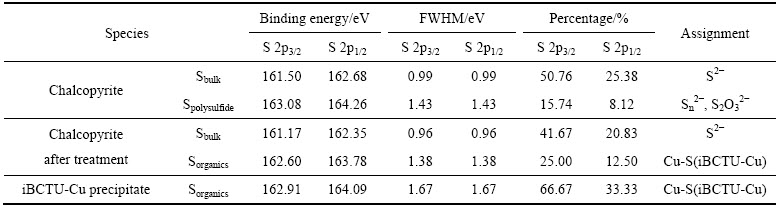
表8 黄铜矿吸附iBCTU前后及iBCTU-Cu沉淀的N 1s XPS峰参数及化学态
Table 8 Deconvolution with Gaussian-Lorentzian bands of N 1s XPS spectra for untreated and treated chalcopyrite, and iBCTU-Cu precipitate
因此,黄铜矿吸附iBCTU前后的XPS结果表明,iBCTU可能通过其硫代羰基S原子和氨基(—NH2)N原子与Cu原子同时成键而吸附在黄铜矿表面,同时将黄铜矿表面的Cu(Ⅱ)还原成Cu(Ⅰ)。据此可以推测,在iBCTU与Cu2+反应或吸附于黄铜矿表面的过程中,其活性官能团C(=O)—NH—C(=S)—NH2重排形成C(=O)—NH—C(—SH)=NH,然后铜原子与硫原子键合成Cu—S键,释放出H+;同时,=NH中的氮原子有富裕电子,也参加与铜原子的成键,形成四元环络合物;当然,C(=O)—NH—C(=S)中的氮原子也可能与黄铜矿表面的铜原子成键[5-6, 14, 16-17]。这样,iBCTU有可能与黄铜矿表面的铜原子形成双四元环络合物。并且,iBCTU的端—NH2没有烃链,使得其更容易靠近黄铜矿表面,与黄铜矿作用时的空间位阻效应较小,有利于其在黄铜矿表面的吸附,从而改善黄铜矿的疏水性,促进黄铜矿上浮,这与浮选和吸附试验结果相一致。
3 结论
1) 浮选和吸附结果表明,在pH 3~11的范围内,iBCTU对黄铜矿具有良好捕收能力,其在黄铜矿表面的吸附量随着温度的升高而增大。热力学分析表明,iBCTU在黄铜矿表面的吸附等温线符合Langmuir模型,吸附焓变ΔH为56.42 kJ/mol, 熵变ΔS为284.76 J/(mol·K),吸附自由能变ΔG为-29.12 kJ/mol (298K),说明iBCTU可能以单分子层化学吸附于黄铜矿表面,吸附为自发、吸热化学吸附过程。
2) XPS的结果表明,iBCTU可能通过其硫代羰基S原子和氨基(—NH2)N原子与Cu原子成键而吸附在黄铜矿表面,同时将黄铜矿表面的Cu(Ⅱ)还原成Cu(Ⅰ)。
REFERENCES
[1] 高学祥, 董 鑫, 花成文. N-二茂铁甲酰基-N'-芳基双( 单) 硫脲类衍生物的合成及生物活性研究[J]. 化学试剂, 2012, 34( 3): 195-198.
GAO Xue-xiang, DONG Xin, HUA Cheng-wen. Synthesis and biological activities of N-ferrocenylformyl-N′-aryl bis-(mono-)thiourea derivatives[J]. Chemical Reagents, 2012, 34(3): 195-198.
[2] HABTU M M, BOURNE S A, KOCH K R. Competitive bulk liquid membrane transport and solvent extraction of some transition and post-transition metal ions using acylthiourea ligands as ionophores[J]. New Journal of Chemistry, 2006, 30: 1155-1162.
[3]  TABANCA N, ALI A, KHAN S I, KHAN I A, WEDGE D E. Synthesis and biological activity of substituted urea and thiourea derivatives containing1,2,4-triazole moieties[J]. Molecules, 2013, 18: 3562-3576.
TABANCA N, ALI A, KHAN S I, KHAN I A, WEDGE D E. Synthesis and biological activity of substituted urea and thiourea derivatives containing1,2,4-triazole moieties[J]. Molecules, 2013, 18: 3562-3576.
[4] BIRINCI E, GULFEN M, AYDIN A O. Separation and recovery of palladium(II) from base metal ions by melamine-formaldehyde-thiourea (MFT) chelating resin[J]. Hydrometallurgy, 2009, 95: 15-21.
[5] 刘广义, 任 恒, 詹金华, 钟宏. 3, 3'-二乙基-1,1'-一缩二乙二醇二羰基双硫脲的合成、表征与性能[J]. 中国有色金属学报, 2013, 23(1): 290-296.
LIU Guang-yi, REN Heng, ZHAN Jin-hua, ZHONG Hong. Synthesis, characterization and properties of 3,3’-diethyl-1,1’-oxydiethylenedicarbonyl bis(thiourea)[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(1): 290-296.
[6] 狄 宁, 肖静晶, 刘广义, 黄志强, 曹占芳, 王 帅. N-丁氧丙基-N′-乙氧羰基硫脲对硫化矿物的浮选行为与吸附机理[J]. 中国有色金属学报, 2014, 24(2): 561-568.
DI Ning, XIAO Jing-jing, LIU Guang-yi, HUANG Zhi-qiang, CAO Zhan-fang, WANG Shuai. Adsorption mechanism and flotation behaviors of N-butoxypropyl-N’-ethoxycarbonyl thiourea with sulfide minerals[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(2): 561-568.
[7] LIU Guang-yi, ZHONG Hong, XIA Liu-yin, WANG Shuai, XU Zheng-he. Improving copper flotation recovery from a refractory copper porphyry ore by using ethoxycarbonyl thiourea as a collector[J]. Minerals Engineering, 2011, 24: 817-824.
[8] LIU Guang-yi, ZHONG Hong, XIA Liu-yin, WANG Shuai, DAI Ta-gen. Effect of N-substituents on performance of thiourea collectors by density functional theory calculations[J]. Transactions of Nonferrous Metals Society of China, 2010, 20(4): 695-701.
[9] 刘广义, 钟 宏, 戴塔根, 夏柳荫. 中碱度条件下乙氧羰基硫脲浮选分离铜硫[J]. 中国有色金属学报, 2009, 19(2): 389-396.
LIU Guang-yi, ZHONG Hong, DAI Ta-gen, XIA Liu-yin. Flotation separation of Cu/Fe sulfide minerals by ethoxycarbonyl thiourea under middle alkaline conditions[J]. The Chinese Journal of Nonferrous Metals, 2009, 19(2): 389-396.
[10] MADDANI M R, PRABHU K R. A concise synthesis of substituted thiourea derivatives in aqueous medium[J]. Journal of Organic Chemistry, 2010, 75(7): 2327-2332.
[11] CAROLINA G O, PEDRO I S M, PAULA S. Manganese(II) complexes with thiosemicarbazones as potential anti-Mycobacterium tuberculosis agents[J]. Journal of Inorganic Biochemistry, 2014, 132: 21-29.
[12] 聂金艳, 司云森, 余 强, 王招娣. N,N-二苯基硫脲对 Q235 钢在酸性介质中的缓蚀作用及其与十二烷基磺酸钠的协同效应[J]. 腐蚀科学与防护技术, 2011, 23(5): 422-426.
NIE Jin-yan, SI Yun-sen, YU Qiang, WANG Zhao-di. Effect of N,N'-diphenylthiourea on corrosion of Q235 steel in acid medium and synergistic effect with sodium dodecyl sulfate[J]. Corrosion Science and Protection Technology, 2011, 23(5): 422-426.
[13] NAGARAJ D R, BASILIO C I, YOON R H. The chemistry and structural-activity relationships for new sulfide collectors[C]// Processing of Complex Ores. Oxford: Pergamon Press, 1989: 157-166.
[14] 刘广义, 张慧丽, 任 恒, 王勇泉, 肖静晶, 高金亮, 范佳志, 钟 宏. N, N′二异丙氧基丙基N″,N'''氧二乙氧羰基硫脲在黄铜矿表面的吸附动力学和热力学特性[J]. 中南大学学报(自然科学版), 2015, 46(5): 1588-1594.
LIU Guang-yi, ZHANG Hui-li, REN Heng, WANG Yong-quan, XIAO Jing-jing, GAO Jin-liang, FAN Jia-zhi, ZHONG Hong. Adsorption kinetics and thermodynamics of N,N′- dipropoxypropyl-N″, N′′′- oxydiethylenedicarbonyl bis(thiourea) with chalcopyrite[J]. Journal of Central South University, 2015, 46(5): 1588-1594.
[15] NAGARAJ D R, LEWELLYN M E, WANG S S. New sulfide and precious metals collectors for acid, neutral and mildly alkaline cricurts[C]//FORSSBERG E. Proeedings of the XVIth International Mineral Proeessing Congress. Amsterdam: Elsevier Science Publisher, 1988: 1221-1232.
[16] LIU Guang-yi, QIU Zhao-hui, WANG Jing-yi, LIU Qing-xia, XIAO Jing-jing, ZENG Hong-bo, ZHONG Hong, XU Zheng-he. Study of N-isopropoxypropyl-N’-ethoxycarbonyl thiourea adsorption on chalcopyrite using in situ SECM, ToF-SIMS and XPS[J]. Journal of Colloid and Interface Science, 2015, 437: 42-49.
[17] XIAO Jing-jing, Di Ning, LIU Guang-yi, ZHONG Hong. The interaction of N-butoxypropyl-N′-ethoxycarbonylthiourea with sulfide minerals: Scanning electrochemical microscopy, diffuse reflectance infrared Fourier transform spectroscopy, and thermodynamics[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2014, 456: 203-210.
[18] 钟 宏, 刘广义, 袁 露. 一种浮选捕收剂及其制备方法: 中国, CN 101698161A[P]. 2010-04-28.
ZHONG Hong, LIU Guang-yi, YUAN-Lu. A kind of flotation collector and its preparation method: China, CN 101698161A[P]. 2010-04-28.
[19] LANGMIUR I. The adsorption of gases on plane surfaces of glass, mica and platinum[J]. Journal of the American Chemistry Society, 1918, 40(9): 1361-1403.
[20] FREUNDLICH H M F. Over the adsorption in solution[J]. Zeitschriftfür Physikalische Chemie, 1906, 57: 385-471.
[21] YANG J B, YU M Q, QIU T. Adsorption thermodynamics and kinetics of Cr(VI) on KIP210 resin[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(2): 480-486.
[22] ZHAO Z W, XU X Y, CHEN X Y, HUO G S, CHEN A L, LIU XH, XU H. Thermodynamics and kinetics of adsorption of molybdenum blue with D301 ion exchange resin[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(3): 686-693.
[23] 曲肖彦, 刘广义, 刘 胜, 钟 宏, 肖静晶. 3-己基-4-氨基-1,2,4-三唑-5-硫酮在黄铜矿表面的吸附动力学与热力学[J]. 中国有色金属学报, 2015, 25(7): 2006-2014.
QU Xiao-yan, LIU Guang-yi, LIU Sheng, ZHONG Hong, XIAO Jing-jing. Adsorption kinetics and thermodynamics of 3-hexyl-4-amino-1,2,4-triazole-5-thione on surface of chalcopyrite[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(7): 2006-2014.
[24] KWAK J, BARD A J. Scanning electrochemical microscopy. Apparatus and two-dimensional scans of conductive and insulating substrates[J]. Analytical Chemistry, 1989, 61(17): 1794-1799.
[25] FAIRTHORNE G, FORNASIERO D, RALSTON J. Formation of a copper-butyl ethoxycarbonyl thiourea complex[J]. Analytica Chimica Acta, 1997, 346(2): 237-248.
[26] HARMER S L, PRATT A R, NESBITT W H, FLEET M E. Sulfur species at chalcopyrite (CuFeS2) fracture surfaces[J]. American. Mineral, 2004, 89: 1026-1032.
[27] HARMER S L, THOMAS J E, FORNASIERO D, GERSON A R. The evolution of surface layers formed during chalcopyrite leaching[J]. Geochimca et Cosmochimica Acta, 2006, 70(17): 4392-4402.
[28] YOSHIDA T, YAMASAKI K, SAWADA S. An X-ray photoelectron spectroscopic study of 2-mercaptobenzothiazole metal complexes[J]. Bulletin of the Chemical Society of Japan, 1979, 52(10): 2908-2912.
[29] YOSHIDA T. An X-ray photoelectron spectroscopic study of several metal complexes of 2-mercaptobenzimidazole and 2-mercaptobenzoxazole[J]. Bulletin of the Chemical Society of Japan, 1980, 53: 1449-1450.
Mechanism of N-isobutoxycarbonyl thiourea (iBCTU) for chalcopyrite flotation
LIU Wei, LIU Guang-yi, XIAO Jing-jing, He Zhi-ling, ZHONG Hong
(School of Chemistry and Chemical Engineering, Central South Univeristy, Changsha 410083, China)
Abstract: A novel surfactant, N-isobutoxycarbonyl thiourea (iBCTU) was synthesized and characterized by infrared spectroscopy, H nuclear magnetic resonance (HNMR) and C nuclear magnetic resonance (CNMR). Its flotation performance, adsorption thermodynamics and mechanism for the chalcopyrite were evaluated. The results of micro-flotation and adsorption tests show that iBCTU exhibits excellent hydrometallurgical performance for chalcopyrite under pH 3-11, and the adsorption capacity increases with increasing temperature. The adsorption isotherm agrees well with Langmuir model and the thermodynamics parameters ΔH (enthalpy change), ΔS (entropy change) and ΔG (free energy change) are 56.42 kJ/mol, 284.76 J/(mol·K) and -29.12 kJ/mol (298K), respectively, which infers that the adsorption of iBCTU on the chalcopyrite surface is a spontaneous and endothermic chemisorption. The XPS results further demonstrate that iBCTU might bond with the copper atoms on the chalcopyrite surface through its sulfur (—C=S) and nitrogen (—NH2) atoms to form iBCTU-Cu(Ⅰ) surface complexes.
Key words: N-isobutoxycarbonyl thiourea; chalcopyrite; adsorption thermodynamics; flotation; XPS
Foundation item: Project(51474253) supported by the National Natural Science Foundation of China; Project (2013AA064101) supported by the National High Technology Research and Development Program of China
Received date: 2015-12-04; Accepted date: 2016-05-16
Corresponding author: LIU Guang-yi; Tel: +86-731-88879616; E-mail: guangyiliu@csu.edu.cn
(编辑 王 超)
基金项目:国家自然科学基金资助项目(51474253);国家高技术研究发展计划资助项目(2013AA064101)
收稿日期:2015-12-04;修订日期:2016-05-16
通信作者:刘广义,教授,博士;电话:0731-88879616;E-mail:guangyiliu@csu.edu.cn