钽在碳酸乙烯酯-氯化铝溶剂化离子液体中的电化学行为
来源期刊:中国有色金属学报(英文版)2020年第8期
论文作者:刘爱民 郭梦霞 吕梓阳 张保国 刘风国 陶文举 杨酉坚 胡宪伟 王兆文 刘玉宝 石忠宁
文章页码:2283 - 2292
关键词:电化学行为;离子液体;循环伏安法;氯化铝;钽
Key words:electrochemical behavior; ionic liquid; cyclic voltammetry; aluminum chloride; tantalum
摘 要:以钨丝为工作电极,通过循环伏安法研究碳酸乙烯酯-氯化铝(EC-AlCl3)溶剂化离子液体中Ta(V)的电化学还原机理。在EC-AlCl3-TaCl5离子液体的循环伏安曲线中出现4个还原峰,其中-0.55、-0.72和-1.12 V(vs Al)处还原峰对应的反应为Ta(V)络合离子三步还原为金属钽,包括Ta(IV)和Ta(III)络合离子的形成。络合离子 Ta(III)被还原为金属钽的过程受扩散控制且不可逆,其在323 K下的扩散系数为3.7×10-7 cm2/s,扩散活化能为 77 kJ/mol。另外,对323 K下的阴极产物进行扫描电子显微镜、能量分散光谱和X射线光电子能谱分析。结果表明,在-0.8 V下恒电位电沉积2 h的产物为金属钽和钽的氧化物。
Abstract: To investigate the electrochemical reduction mechanism of Ta(V) in ethylene carbonate and aluminum chloride (EC-AlCl3) solvate ionic liquid, cyclic voltammetry experiments were conducted on a tungsten working electrode. Four reduction peaks were observed in the cyclic voltammogram of the EC-AlCl3-TaCl5 ionic liquid. The reduction peaks at -0.55, -0.72, and -1.12 V (vs Al) were related to the reduction of Ta(V) to tantalum metal by three stages including the formation of Ta(IV) and Ta(III) complex ions. The reduction of Ta(III) to tantalum metal was an irreversible diffusion-controlled reaction with a diffusion coefficient of 3.7×10-7 cm2/s at 323 K, and the diffusion activation energy was 77 kJ/mol. Moreover, the cathode products at 323 K were characterized by scanning electron microscopy, energy-dispersive spectroscopy, and X-ray photoelectron spectroscopy. The results showed that tantalum metal and tantalum oxides were obtained by potentiostatic electrodeposition at -0.8 V for 2 h.
Trans. Nonferrous Met. Soc. China 30(2020) 2283-2292
Ai-min LIU1, Meng-xia GUO1, Zi-yang Lü1, Bao-guo ZHANG1, Feng-guo LIU1, Wen-ju TAO1, You-jian YANG1, Xian-wei HU1, Zhao-wen WANG1, Yu-bao LIU2, Zhong-ning SHI3
1. Key Laboratory for Ecological Metallurgy of Multimetallic Mineral, Ministry of Education, Northeastern University, Shenyang 110819, China;
2. State Key Laboratory of Baiyunobo Rare Earth Resource Researches and Comprehensive Utilization, Baotou Research Institute of Rare Earths, Baotou 014030, China;
3. State Key Laboratory of Rolling and Automation, Northeastern University, Shenyang 110819, China
Received 20 September 2019; accepted 28 June 2020
Abstract: To investigate the electrochemical reduction mechanism of Ta(V) in ethylene carbonate and aluminum chloride (EC-AlCl3) solvate ionic liquid, cyclic voltammetry experiments were conducted on a tungsten working electrode. Four reduction peaks were observed in the cyclic voltammogram of the EC-AlCl3-TaCl5 ionic liquid. The reduction peaks at -0.55, -0.72, and -1.12 V (vs Al) were related to the reduction of Ta(V) to tantalum metal by three stages including the formation of Ta(IV) and Ta(III) complex ions. The reduction of Ta(III) to tantalum metal was an irreversible diffusion-controlled reaction with a diffusion coefficient of 3.7×10-7 cm2/s at 323 K, and the diffusion activation energy was 77 kJ/mol. Moreover, the cathode products at 323 K were characterized by scanning electron microscopy, energy-dispersive spectroscopy, and X-ray photoelectron spectroscopy. The results showed that tantalum metal and tantalum oxides were obtained by potentiostatic electrodeposition at -0.8 V for 2 h.
Key words: electrochemical behavior; ionic liquid; cyclic voltammetry; aluminum chloride; tantalum
1 Introduction
Biomedical metallic materials are widely used in medical fields such as orthopedics, dentistry, and cardiovascular stent intervention. Tantalum is an ideal medical material with good corrosion resistance and biocompatibility [1], but its expensive price limits its wide application. To reduce the cost of tantalum metal, it is feasible and promising to prepare metallic tantalum coatings on the surface of a relatively inexpensive and easy processing alloy.
The chemical vapor deposition (CVD) is an important method for surface coating preparation. YU et al [2] studied the effects of the hydrogen flow rate and deposition temperature on the surface quality of tantalum coatings prepared by CVD. In addition, tantalum coatings can be prepared by magnetron sputtering and arc ion plating [3-5]. MAENG et al [3] produced tantalum coatings with good corrosion resistance by cylindrical magnetron sputtering on the surface of AISI 4340 steel. CHENG et al [5] used the arc ion plating technique to prepare tantalum on the surface of a Ni-Ti alloy, and the corrosion current density in a 0.9% NaCl solution at 310 K was 0.75 μA/cm2. The tantalum coating prepared by the above methods can improve the corrosion resistance and biocompatibility of conventional medical metal substrates, but it is difficult to accommodate the internal and dead angles of irregularly shaped matrix materials.
Electrodeposition in room temperature ionic liquid is a new idea for preparing tantalum coatings on the surface of traditional biomedical metallic materials. ABEDIN et al [6,7] prepared tantalum coatings with a thickness of 300 nm on Pt and Au(111) substrates from a 1-butyl-1-methylpyrrole bistrifluoromethylsulfonimide ([BMP]Tf2N) ionic liquid containing 0.25 mol/L LiF and 0.5 mol/L TaF5 at 473 K. NAHRA et al [8] studied the electrochemical reduction of TaF5 in a 1-butyl- 3-methyl pyrrolidinium bis(trifluoromethyl sulfonyl) imide ([BMP]TFSI) ionic liquid. The results showed that TaF5 was reduced to metallic tantalum at potentials from -0.9 to -2.0 V (vs Pt), and the reduction peaks appearing more negative than -2.0 V (vs Pt) were ascribed to the reduction of TaF6- or TaF72- complex ions. Moreover, 1-butyl-1-methylpyrrole bistrifluoromethylsulfon- amide ([BMP]TFSA) ionic liquid was considered to be suitable for tantalum electrodeposition due to its wide electrochemical window (5.6 V) [9]. When 0.25 mol/L LiF is added to the [BMP]TFSA ionic liquid, a tantalum coating with a thickness of 1 μm can be deposited on the stainless steel substrate. BORISENKO et al [10] investigated the electro- chemical behavior of TaF5 in [BMP]TFSA. The results demonstrated that TaF5 was reduced to TaF3 at -1.0 V (vs Pt), while TaF3 was reduced to TaF2, TaF1.5, Ta2F and metallic tantalum at potentials between -1.2 and -2.3 V (vs Pt). ISPAS et al [11] found that tantalum could not be deposited in [EMIm]TFSA ionic liquid, whereas the electro- deposition of tantalum in [BMP]TFSA ionic liquid was feasible at room temperature, and the applied potential ranged from -2.0 to -2.3 V (vs Pt). In addition, MAIS et al [12] synthesized Cu-Ta deposits from [BMP]TFSA-TaF5-LiF ionic liquid at -1.8 V (vs Pt) and 398 K.
Studies were also carried out using TaCl5 as a starting material to electrodeposit tantalum coatings at low temperature. BARNARD et al [13] studied the mechanism of tantalum electrodeposition in 1-methyl-3-ethylimidazolium chloride-aluminum chloride (MeEtImCl-AlCl3) ionic liquid at 313 K. The results indicated that TaCl5 combined with Cl- to form TaCl6-, and the electrochemical reduction of TaCl5 proceeded by two steps, namely, Ta(V)→ Ta(III)→Ta. SATO et al [14] prepared an Al-Ta alloy with a maximum Ta content of 72% from NaCl-KCl-AlCl3 melts at 423 K. JO et al [15] produced nanoparticles of tantalum (~10 nm) on the surface of carbon black from acetonitrile at room temperature. XIE et al [16] reported that the electrodeposition of tantalum in a 1-butyl-3-methylimidazolium hexafluorophosphate ([BmIm]PF6) ionic liquid was a two-step reaction, namely, Ta(V)→Ta(III)→Ta. The products obtained by potentiostatic electrodeposition at -1.25 V (vs Pt) were mixtures of tantalum and tantalum subchloride. Although tantalum can be electrodeposited from the abovementioned ionic liquids, oxofluoride complexes of Ta(V) and subvalent metal halide clusters such as TaOCl4- and  were found to prevent the continuous electrodeposition of tantalum. In addition, the above ionic liquids are relatively expensive, which greatly limits the commercial promotion and industrial production of tantalum coatings.
were found to prevent the continuous electrodeposition of tantalum. In addition, the above ionic liquids are relatively expensive, which greatly limits the commercial promotion and industrial production of tantalum coatings.
Recently, our research group has developed a novel room temperature solvate ionic liquid consisting of ethylene carbonate and aluminum chloride (EC-AlCl3), which exhibits the advantages of low cost, high stability, and good solubility of metal chlorides. This system is suitable for electro- deposition of active materials such as Al, Li, and Al-Nd alloys under ambient conditions [17-19]. However, few studies have been devoted to the electrodeposition of tantalum in solvate ionic liquids. In this work, the feasibility and electrochemical mechanism of tantalum electro- deposition in the EC-AlCl3 solvate ionic liquid was thoroughly explored.
2 Experimental
Ethylene carbonate (EC, 98 wt.%), aluminum chloride (AlCl3, 99 wt.%), and tantalum chloride (TaCl5, 95 wt.%) were purchased from Shanghai Aladdin Bio-Chem Technology Company Limited, China. The preparation of the ionic liquid was carried out in a glove box (MB 200B, Braun, Germany) filled with argon gas (99.99%), wherein the contents of water and oxygen were less than 0.1 μg/g. Firstly, certain amounts of EC and AlCl3 (molar ratio 1:0.2) were ground into powders, respectively. Secondly, the AlCl3 powder was slowly added to a beaker filled with EC powder, and a glass rod was used to stir continuously until the powders were evenly mixed. Then, a clear and transparent solution was obtained and placed on a magnetic heating plate. Finally, the EC-AlCl3- TaCl5 (molar ratio 1:0.2:0.003) solvate ionic liquid was prepared by adding TaCl5 powder into the EC-AlCl3 solution with magnetic stirring.
The electrochemical experiments were conducted in a glove box using an electrochemical workstation (CHI660E, Shanghai Chenhua Instrument Company Limited). Two tungsten wires (99.95%) were used as the working electrode and counter electrode, while an aluminum wire (99.99%) was used as the reference electrode. Prior to electrochemical measurements, each electrode was polished with different sizes of sandpapers and washed with ethanol.
The electrodeposition experiments were also performed in the glove box using an electrochemical workstation with a three-electrode system. The working electrode and counter electrode were tungsten sheets (99.95%), while the reference electrode was an aluminum wire. After the electrodeposition experiment, the working electrode was washed with acetonitrile, ethanol, and deionized water to remove the remaining electrolytes on the surface of the tungsten sheet. In addition, the surface morphology of the product was observed using scanning electron microscopy (SEM, EVO18, Carl Zeiss, Germany). Energy- dispersive spectrometer (EDS, X-Max-80, Oxford, UK), which was combined with the scanning electron microscopy system, was used to semi- quantitatively determine the elemental contents of the deposits. The chemical state of tantalum in the deposits was characterized by X-ray photoelectron spectroscopy (XPS, Axis Ultra DLD, Kratos, Japan).
3 Results and discussion
3.1 Cyclic voltammetry curves of EC-AlCl3 ionic liquid
Cyclic voltammetry (CV) was carried out to study the electrochemical behavior of Al(III) ions in the EC-AlCl3 solvate ionic liquid. The CV curves of EC and the EC-AlCl3 solvate ionic liquid at 323 K are shown in Fig. 1. There was no reduction or oxidation peak at potentials scanned from 1.5 to -2.0 V (vs Al) in the CV curve of the EC solutions, indicating that the electrolyte was electrochemically stable within this potential range. When AlCl3 was added to the EC solutions, the CV curve (as seen in the blue curve of Fig. 1), which recorded over a potential range from 1.5 to 0.4 V (vs Al) demonstrated an apparent reduction peak at 0.53 V (vs Al), and the corresponding oxidation peak was found at 0.63 V during the reverse sweep. When the CV curve (as seen in the red curve of Fig. 1) was measured over a potential range from 1.5 to -0.9 V (vs Al), it is interesting that two reduction peaks were detected at 0.53 and -0.75 V (vs Al); in the backward scan, two oxidation peaks were found at 0.63 and 0.27 V (vs Al), respectively. Compared with the CV curve of the EC solution, two pairs of redox peaks in the CV curve of the EC-AlCl3 solvate ionic liquid were believed to involve the redox reaction of Al(III) complex ions.
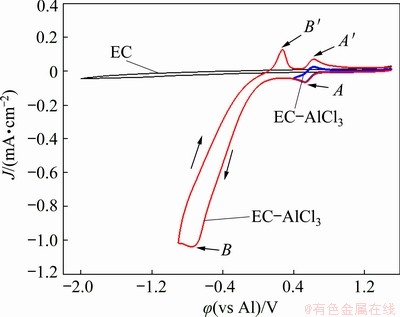
Fig. 1 Cyclic voltammograms of EC and EC-AlCl3 (molar ratio 1:0.2) solvate ionic liquid at 323 K and scan rate of 60 mV/s
According to the findings of research conducted by ZHANG et al [17], the EC-AlCl3 solvate ionic liquid primarily consists of the AlCl4- anion, charge-neutral [Al3(EC)2] complexes, and [AlCl2(EC)4]+ complex cation. Furthermore, it was suggested that metallic aluminum was formed by electrochemical reduction of the [AlCl2(EC)4]+ complex cation, which can be represented by the equation 2[AlCl2(EC)4]++3e→Al+AlCl4-+8EC. However, two pairs of apparent reduction and oxidation peaks were found in the CV curve of the EC-AlCl3 solvate ionic liquid (molar ratio 1:0.2). Considering the studies of CARLIN et al [20], LEE et al [21], and JIANG et al [22], a pair of minor peaks corresponding to the underpotential deposition (UPD) of aluminum can be found at a potential more positive than that of bulk aluminum deposition in the AlCl3-1-ethyl-3-methyl imidazolium chloride ([EMIm]Cl) ionic liquid.
Therefore, the minor redox peaks A and A' (Fig. 1) were related to the UPD and anodic stripping of aluminum on the tungsten electrode. Similarly, the UPD of aluminum from AlCl3-based electrolytes was reported by ZHAO et al [23] in aluminum chloride-trimethyl phenylammonium chloride and by ZELL et al [24] in aluminum chloride-1-methyl-3-butylimidazolium chloride ionic liquid.
3.2 Electrochemical behavior of Ta(V) in EC- AlCl3-TaCl5 ionic liquid
To study the electrochemical reduction of Ta(V) complex ions in the EC-AlCl3-TaCl5 (molar ratio 1:0.2:0.003) solvate ionic liquid at 323 K, a cyclic voltammetry curve was recorded at a scan rate of 60 mV/s using a tungsten wire as the working electrode. As shown in Fig. 2, the CV curve of the EC-AlCl3-TaCl5 (molar ratio 1:0.2:0.003) solvate ionic liquid was compared with that of EC and EC-AlCl3 (molar ratio 1:0.2) electrolytes.

Fig. 2 Cyclic voltammograms of EC, EC-AlCl3 (molar ratio 1:0.2), and EC-AlCl3-TaCl5 (molar ratio 1:0.2:0.003) solvate ionic liquid at 323 K and scan rate of 60 mV/s
It can be seen from Fig. 2 that there were no redox peaks in the CV curve of the EC system, indicating that the EC solution was very stable within the potential range from 1.5 to -2.0 V (vs Al). When AlCl3 was added into the EC solutions, apparent redox peaks were detected and assigned to the bulk deposition of aluminum. The current density of the aluminum reduction peak was approximately 1.05 mA/cm2, which was much smaller than that of the reduction peaks in the EC-AlCl3 ionic liquid containing TaCl5 (~8.06 mA/cm2). The current signal of aluminum reduction disappeared in the CV curve of the EC-AlCl3-TaCl5 solvate ionic liquid due to the reaction of [AlCl2(EC)4]+ cations with TaCl5 to form [TaxClm(EC)n]5x-m complex electroactive species [17,25]. Notably, four reduction peaks appeared at -0.20, -0.55, -0.72, and -1.12 V (vs Al). Obviously, these reduction peaks were believed to involve the redox reaction of Ta(V) complex ions.
According to Refs. [7,12], TaF3 species are stable in TaF5-containing ionic liquids such as [BMP]TFSA-TaF5, and two reduction peaks were observed in the CV curve, indicating that the electrochemical reduction of Ta(V) was a two-step mechanism including the reduction of Ta(V) complex ions to Ta(III) species. In addition, four reduction peaks were observed by BABUSHKINA and SILVIA [26] in the CV curve of the 1-butyl- 1-methylpyrrolidinium chloride (Pyr14Cl)-TaCl5 ionic liquid. It was suggested that the first reduction peak was assigned to the reduction of oxochloride complexes of Ta(V) to tantalum oxides. The second reduction peak was attributed to the reduction of Ta(V) ([TaCl6]-) to Ta(IV) ([TaCl6]2-) complex ions, while the third and fourth reduction peaks were related to the reduction of Ta(IV) ([TaCl6]2-) to Ta(III) ([TaCl6]3-) complex ions and the further reduction of Ta(III) complex ions to metallic tantalum, respectively. Therefore, the reduction peak observed at -0.20 V (vs Al) in the CV curve of EC-AlCl3-TaCl5 (peak C in Fig. 2) was due to the reduction of Ta(V) chloride complexes, while the oxidation peak C' was assigned to the irreversible oxidation of Cl- anion with Cl2 evolution [16,26]. Furthermore, the reduction peaks at -0.55, -0.72, and -1.12 V may be attributed to the reduction of Ta(V) complex ions to metallic tantalum by a three-step process including the electrolytic formation of Ta(IV) and Ta(III) complex ions. In the reverse scan in Fig. 2, the oxidation peaks F', E', and D' can be ascribed to the oxidation of metallic tantalum to Ta(III), Ta(IV), and Ta(V) complex ions, respectively.
Figure 3 presents the cyclic voltammograms of the EC-AlCl3-TaCl5 solvate ionic liquid at 323 K and different scan rates. The scan rates were set as 40, 60, 80, 100, 120, and 140 mV/s. According to the above discussion, the reduction peaks D and E in Fig. 3 corresponded to the reduction of Ta(V) to Ta(IV) and the subsequent reduction of Ta(IV) to Ta(III), respectively, while the reduction peak F was due to the reduction of Ta(III) to tantalum. As the scan rates increased from 40 to 140 mV/s, the potentials of the reduction peaks moved toward more negative values, while the potentials of the oxidation peaks moved toward more positive values. In addition, the current densities of the reduction peaks increased with increasing scan rates, indicating that the reduction of Ta(III) complex ions to metallic tantalum was an irreversible process controlled by diffusion [26].
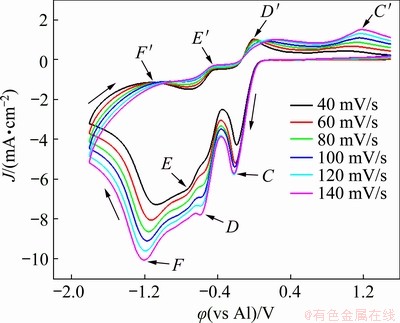
Fig. 3 Cyclic voltammograms of EC-AlCl3-TaCl5 (molar ratio 1:0.2:0.003) solvate ionic liquid at 323 K and different scan rates of 40, 60, 80, 100, 120, and 140 mV/s
For an irreversible electrode reaction, the transfer coefficient was linearly related to the differences between the reduction peak potentials and the reduction half-peak potentials, which can be presented as Eq. (1) [27]:
|φp-φp/2| =1.857RT/(αnF) (1)
where φp is the potential of the reduction peak (V), φp/2 is the potential of the reduction half-peak (V), R is the mole gas constant (8.314 J/(K·mol)), T is the thermodynamic experimental temperature (323 K), α is the transfer coefficient, n is the number of electrons transferred during the reduction reaction, and F is the Faraday constant (96500 C/mol).
According to Eq. (1) and data derived from reduction peak F (Ta(III)/Ta) in the cyclic voltammograms of the EC-AlCl3-TaCl5 solvate ionic liquid in Fig. 3 at different scan rates (Table 1), the average value of transfer coefficient α was calculated to be 0.2 at 323 K. Moreover, the diffusion coefficient (D) of the tantalum complex species during the electrochemical reduction process can be determined according to the Randles-Sevcik equation [27]:
Jp=0.4958nFC0(αnF/RT)1/2D1/2v1/2 (2)
where Jp is the current density of the reduction peak (A/cm2), C0 is the bulk concentration of tantalum complex species (mol/mL), D is the diffusion coefficient of tantalum complex species (cm2/s), and v is the potential scan rate (V/s).
Table 1 Data derived from reduction peak F (Ta(III)/Ta) in cyclic voltammograms of EC-AlCl3-TaCl5 (Fig. 3) at different scan rates

It can be demonstrated from the inset curves of Fig. 4 that there is a linear correlation between the current density of reduction peak F (Jp) and the square root of the scan rate (v1/2), suggesting that the reduction of Ta(III) complex ions to metallic tantalum was a diffusion-controlled process. Therefore, the values of Jp/v1/2 at 323 K can be obtained from the slope of the inset curves of Fig. 4. Substituting these data into Eq. (2), the diffusion coefficient of tantalum complex species in the EC-AlCl3-TaCl5 (molar ratio 1:0.2:0.003) solvate ionic liquid at 323 K was determined to be 3.7×10-7 cm2/s.
Furthermore, cyclic voltammetry experiments of the EC-AlCl3-TaCl5 (molar ratio 1: 0.2: 0.003) solvate ionic liquid were carried out at different temperatures ranging from 303 to 333 K (as shown in Fig. 4). Based on the Randles-Sevcik equation, the diffusion coefficients of tantalum complex species at 303, 313, and 333 K were calculated to be 0.6×10-7, 1.5×10-7, and 9.7×10-7 cm2/s, respectively, which was mainly caused by the decreased viscosity of the solvate ionic liquid with increasing experimental temperature [28].

Fig. 4 Cyclic voltammograms of EC-AlCl3-TaCl5 (molar ratio 1:0.2:0.003) solvate ionic liquid at different temperatures
According to the Arrhenius equation, the logarithm of the diffusion coefficient (ln D) of tantalum complex species in the EC-AlCl3-TaCl5 (molar ratio 1: 0.2: 0.003) solvate ionic liquid was linearly related to the reciprocal of temperature (T-1), which can be presented as Eq. (3) [29]:
ln D=ln D0-Ea/(RT) (3)
where D is the diffusion coefficient (cm2/s), D0 is the frequency factor (cm2/s), and Ea is the diffusion activation energy (J/mol). The diffusion coefficient (ln D) of the tantalum complex species in the EC-AlCl3-TaCl5 solvate ionic liquid at temperatures of 303-333 K was calculated according to the Randles-Sevcik equation. Therefore, the value of the diffusion activation energy Ea can be obtained from the slope of Fig. 5. Substituting these data into Eq. (3), the diffusion activation energy of the tantalum complex species was determined to be 77 kJ/mol.
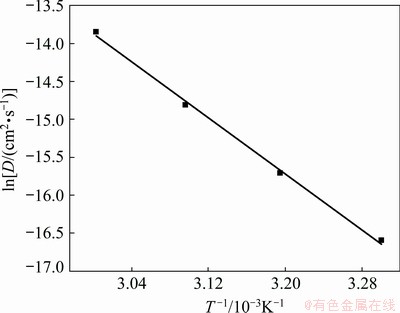
Fig. 5 Natural logarithm of diffusion coefficient ln D of tantalum complex species in EC-AlCl3-TaCl5 (molar ratio 1:0.2:0.003) solvate ionic liquid as function of reciprocal of temperature
3.3 Electrodeposition of Ta in EC-AlCl3-TaCl5 ionic liquid
According to the results from cyclic voltammetry (Fig. 2), the reduction peaks at -0.55 and -0.72 V (vs Al) corresponded to the reduction of Ta(V) to Ta(IV) and the subsequent reduction of Ta(IV) to Ta(III), respectively, while the reduction peak at -1.12 V (vs Al) was due to the reduction of Ta(III) to tantalum. To study the feasibility of tantalum electrodeposition in the EC-AlCl3-TaCl5 electrolyte, experiments were carried out at various potentials stepped from -0.4 to -1.6 V (vs Al) for 2 h. The SEM images and corresponding EDS spectra of the electrodeposits on the surface of tungsten sheets are shown in Figs. 6 and 7, respectively.
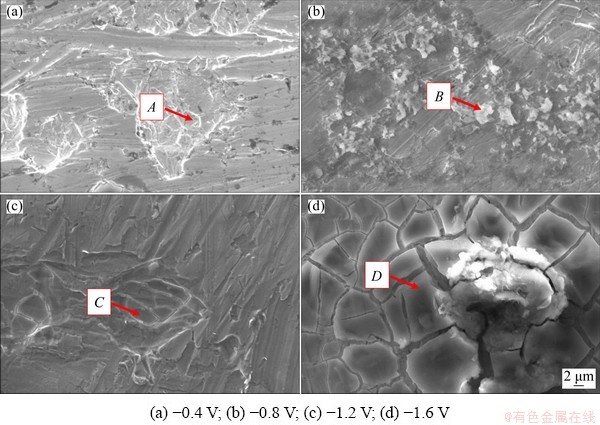
Fig. 6 SEM images of electrodeposits obtained from EC-AlCl3-TaCl5 solvate ionic liquid on surface of tungsten sheets at 323 K for 2 h at different potentials
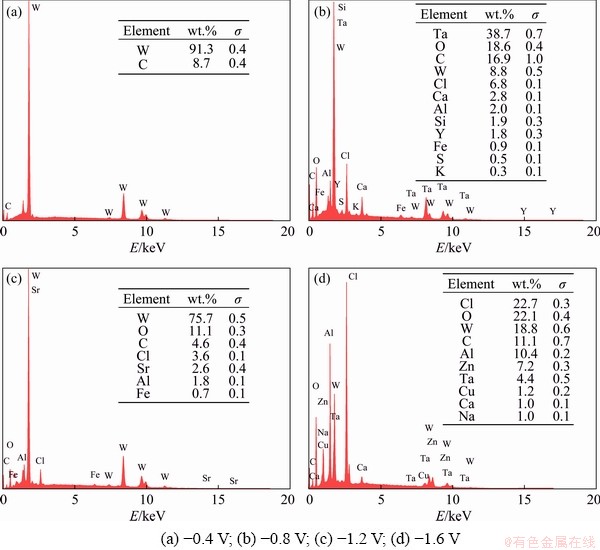
Fig. 7 EDS spectra of electrodeposits obtained from EC-AlCl3-TaCl5 solvate ionic liquid at 323 K for 2 h (σ stands for standard deviation)
When the applied cathode potential was -0.4 V (vs Al), the tungsten substrate (working electrode) showed signs of corrosion due to the acidic EC-AlCl3-TaCl5 solvate ionic liquid, and no metallic tantalum was deposited on the tungsten electrode (Fig. 7(a)). When the applied cathode potential was -1.2 V (vs Al), the tungsten substrate was seriously corroded (Fig. 7(c)). The EDS spectrum in Fig. 7(c) demonstrated that the main component of the product was tungsten, and no metallic tantalum was formed on the tungsten substrate. When the applied cathode potential reached -1.6 V (vs Al), it can be seen that the tungsten substrate was corroded more seriously, and the surface of the substrate was divided into a number of cracks (Fig. 7(d)). Moreover, EDS point analysis in Fig. 7(d) indicated that the deposits consisted of chloride, oxygen, tungsten, carbon, aluminum, zinc and a small quantity of tantalum. The existence of oxygen was possibly caused by ambient air oxidation after the electrodeposition experiment. Although signals of aluminum and tantalum were detected on the surface of the tungsten substrate, the mass fraction of chloride was 22.7%, indicating that the aluminum and tantalum were mainly derived from residual EC-AlCl3-TaCl5 electrolyte.
When the applied cathode potential was -0.8 V (vs Al), small and irregularly shaped particles were formed on the surface of the tungsten substrate, but the coating was extremely sparse and nonuniformly distributed. It can be seen from the EDS spectrum (Fig. 7(b)) that the mass fraction of tantalum was as high as 38.7%, and the mass fraction of aluminum was 2.0%. In addition, the mass fraction of oxygen was determined to be 18.6%, which may be due to the formation of tantalum oxide intermediates [30]. It is worth noting that the chloride mass fraction of the deposits was 6.8%, and the molar ratio of tantalum to chloride was calculated to be 1.1:1, indicating that the electrodeposits at -0.8 V (vs Al) were mixtures of metallic tantalum and tantalum complexes.
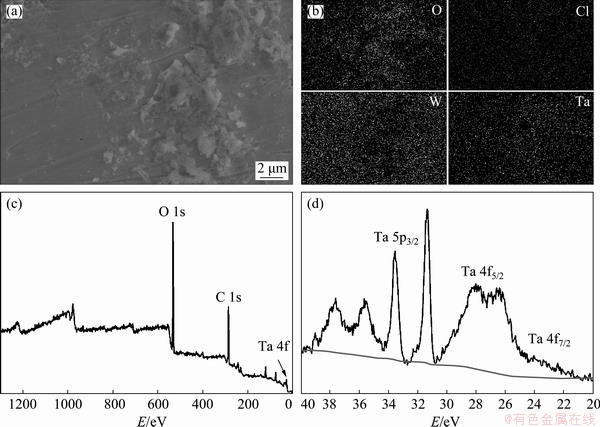
Fig. 8 SEM (a) and corresponding EDS mapping (b) images of electrodeposits obtained from EC-AlCl3-TaCl5 solvate ionic liquid on tungsten substrates at 323 K and -0.8 V (vs Al) for 2 h, XPS spectrum of electrodeposits (c) and high-resolution XPS spectrum of Ta 4f and Ta 5p peaks (d)
Figures 8(a, b) show SEM, corresponding EDS mapping images of electrodeposits obtained from EC-AlCl3-TaCl5 solvate ionic liquid on tungsten substrates at 323 K and -0.8 V (vs Al), respectively. As shown in Fig. 8(b), elements O, Cl, W, and Ta were dispersed throughout the coating obtained by electrodeposition at -0.8 V (vs Al) and 323 K for 2 h. The XPS spectra of the electrodeposits are shown in Figs. 8(c) and (d). It is noted that no Cl could be detected in the XPS spectrum, and the peaks occurring around the binding energies of 532 and 27 eV confirmed existence of the elements O and Ta. Furthermore, the binding energies corresponding to Ta 4f were between 22 and 30 eV, suggesting that tantalum was either metallic tantalum or tantalum bound to oxygen (probably Ta2O5, TaO2, and TaO).
According to the findings of the research conducted by KERREC et al [30] and ISPAS et al [11], tantalum can be oxidized and form compounds with oxygen if the tantalum coating is exposed to air. The formation of tantalum oxide intermediates led to the passivation of the electrode, which prevented the diffusion of tantalum species [TaxClm(EC)n]5x-m and the subsequent electrolytic formation of metallic tantalum. Thus, it is extremely difficult to prepare uniform and compact tantalum coatings by electrodeposition in EC- AlCl3-TaCl5 (molar ratio 1:0.2:0.003) solvate ionic liquid. To the best of our knowledge, the electrodeposition of tantalum in ionic liquid may achieve success via induced codeposition with transition metals such as copper, cobalt, and nickel [12,31,32].
4 Conclusions
(1) Two reduction peaks appeared at 0.53 and -0.75 V (vs Al) in the cyclic voltammogram of the EC-AlCl3 (molar ratio 1:0.2) ionic liquid, which correspond to the underpotential deposition of aluminum and the deposition of bulk aluminum, respectively.
(2) Four reduction peaks were observed in the cyclic voltammogram of the EC-AlCl3-TaCl5 (molar ratio 1:0.2:0.003) solvate ionic liquid. The reduction peaks at -0.55, -0.72, and -1.12 V (vs Al) were ascribed to the reduction of Ta(V) to Ta(IV), the subsequent reduction of Ta(IV) to Ta(III), and the further reduction of Ta(III) to tantalum metal, respectively.
(3) The reduction of Ta(III) to tantalum metal is an irreversible reaction controlled by diffusion, and the diffusion coefficient increased from 0.6×10-7 to 9.7×10-7 cm2/s as the temperature increased from 303 to 333 K.
(4) Electrodeposition experiments showed that the deposits obtained on the surface of the tungsten substrate by electrodeposition at -0.8 V (vs Al) for 2 h were tantalum and tantalum oxides.
References
[1] MIAO Jing-lei, LIU Jue, WANG Hui-feng, YANG Hai-lin, RUAN Jian-ming. Preparation of porous Ta-10%Nb alloy scaffold and its in vitro biocompatibility evaluation using MC3T3-E1 cells [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 2053-2061.
[2] YU Xiao-ming, TAN Li-li, YANG Ke. Preparation and properties of biomedical tantalum coating [J]. Rare Metal Materials and Engineering, 2014, 43: 105-109. (in Chinese)
[3] MAENG S, AXE L, TYSON T A, COTE P. Corrosion behaviour of electrodeposited and sputtered Cr coatings and sputtered Ta coatings with α and β phases [J]. Surface and Coatings Technology, 2006, 200: 5767-5777.
[4] HALLMANN L, ULMER P. Effect of sputtering parameters and substrate composition on the structure of tantalum thin films [J]. Applied Surface Science, 2013, 282: 1-6.
[5] CHENG Y, CAI W, LI H T, ZHENG Y F, ZHAO L C. Surface characteristics and corrosion resistance properties of TiNi shape memory alloy coated with Ta [J]. Surface and Coatings Technology, 2004, 186: 346-352.
[6] ABEDIN S Z E, WELZ-BIERMANN U, ENDRES F. A study on the electrodeposition of tantalum on NiTi alloy in an ionic liquid and corrosion behaviour of the coated alloy [J]. Electrochemistry Communications, 2005, 7: 941-946.
[7] ABEDIN S Z E, FARAG H K, MOUSTAFA E M, WELZ-BIERMANN U, ENDRES F. Electroreduction of tantalum fluoride in a room temperature ionic liquid at variable temperatures [J]. Physical Chemistry Chemical Physics, 2005, 7: 2333-2339.
[8] NAHRA M, SVECOVA L, CHAINET E. Pentavalent tantalum reduction mechanism from 1-butyl-3-methyl- pyrrolidinium bis(trifluoromethylsulfonyl)imide ionic liquid [J]. Electrochimica Acta, 2015, 182: 891-899.
[9] ABEDIN Z E S. Electrodeposition of tantalum and aluminium in ionic liquid [Py1,4]TFSA [J]. Transactions of the IMF, 2008, 86: 220-226.
[10] BORISENKO N, ISPAS A, ZSCHIPPANG E, LIU Q, ZEIN EI ABEDIN S, BUND A, ENDRES F. In situ STM and EQCM studies of tantalum electrodeposition from TaF5 in the air- and water-stable ionic liquid 1-butyl-1- methylpyrrolidinium bis(trifluoromethylsulfonyl) amide [J]. Electrochimica Acta, 2009, 54: 1519-1528.
[11] ISPAS A, ADOLPHI B, BUND A, ENDRES F. On the electrodeposition of tantalum from three different ionic liquids with the bis(trifluoromethylsulfonyl)amide anion [J]. Physical Chemistry Chemical Physics, 2010, 12: 1793-1803.
[12] MAIS L, MASCIA M, VACCA A, PALMAS S, DELOGU F. Electrochemical deposition of Cu and Ta from pyrrolidinium based ionic liquid [J]. Journal of Applied Electrochemistry, 2015, 45: 735-744.
[13] BARNARD P A, HUSSEY C L. Electrochemistry of tantalum(V) chloride in the basic aluminum chloride-l- methyl-3-ethylimidazolium chloride room-temperature molten salt [J]. Journal of the Electrochemical Society, 1990, 137: 913-918.
[14] SATO K, MATSUSHIMA H, UEDA M. Electrodeposition of Al-Ta alloys in NaCl-KCl-AlCl3 molten salt containing TaCl5 [J]. Applied Surface Science, 2016, 388: 794-798.
[15] JO A, LEE Y, LEE C. Electrodeposition of tantalum on carbon black in non-aqueous solution and its electrocatalytic properties [J]. Analytica Chimica Acta, 2016, 933: 59-65.
[16] XIE Fan-xia, HE Xue-ming, LU Yan-ming, YU Jing-hu, WU Mei-ping. Electrochemical behavior of tantalum chloride in ionic liquid 1-butyl-3-methylimidazole hexafluorate phosphate [J]. China Journal of Applied Chemistry, 2016, 33: 1093-1098. (in Chinese)
[17] ZHANG Bao-guo, SHI Zhong-ning, SHEN Ling-ling, LIU Ai-min, XU Jun-li, HU Xian-wei. Electrodeposition of Al, Al-Li alloy, and Li from an Al-containing solvate ionic liquid under ambient conditions [J]. Journal of the Electrochemical Society, 2018, 165: D321-D327.
[18] ZHANG Bao-guo, YAO Yu, SHI Zhong-ning, XU Jun-li, WANG Zhao-wen. Direct electrochemical deposition of lithium from lithium oxide in a highly stable aluminium- containing solvate ionic liquid [J]. Chem Electro Chem, 2018, 5: 3368-3372.
[19] ZHANG Bao-guo, SHI Zhong-ning, SHEN Ling-ling, LIU Xiao-zhen, WANG Zhao-wen. Low-temperature electro- chemical codeposition of aluminum-neodymium alloy in a highly stable solvate ionic liquid [J]. Journal of Solid State Electrochemistry, 2019, 23: 1903-1909.
[20] CARLIN B T, RICHARD T, CRAWFORD W, BERSCH M. Nucleation and morphology studies of aluminum deposited from an ambient-temperature chloroaluminate molten salt [J]. Journal of the Electrochemical Society, 1992, 139: 2720-2727.
[21] LEE J J, BAE I T, SHERSON D A, MILLER B, WHEELER K A. Underpotential deposition of aluminum and alloy formation on polycrystalline gold electrodes from AlCl3/EMIC room-temperature molten salts [J]. Journal of the Electrochemical Society, 2000, 147: 562-566.
[22] JIANG T, CHOLLIER BRYM M J, DUBE G, LASIA A, BRISARD G M. Electrodeposition of aluminium from ionic liquids: Part I—Electrodeposition and surface morphology of aluminium from aluminium chloride (AlCl3)–1-ethyl-3- methylimidazolium chloride ([EMIm]Cl) ionic liquids [J]. Surface and Coatings Technology, 2006, 201: 1-9.
[23] ZHAO Yu-guang, VANDERNOOT T J. Electrodeposition of aluminium from room temperature AlCl3-TMPAC molten salts [J]. Electrochimica Acta, 1997, 42: 1639-1643.
[24] ZELL C A, ENDRES F, FREYLAND W. Electrochemical in situ STM study of phase formation during Ag and Al electrodeposition on Au(111) from a room temperature molten salt [J]. Physical Chemistry Chemical Physics, 1999, 1: 697-704.
[25] ABOOD H M, ABBOTT A P, BALLANTYNE A D, RYDER K S. Do all ionic liquids need organic cations? Characterisation of [AlCl2·namide]+AlCl4- and comparison with imidazolium based systems [J]. Chemical Communications, 2011, 47: 3523-3525.
[26] BABUSHKINA O B, SILVIA E. Spectroscopic study of the electrochemical behaviour of tantalum(V) chloride and oxochloride species in 1-butyl-1-methylpyrrolidinium chloride [J]. Electrochimica Acta, 2010, 56: 867-877.
[27] BARDA J, FAULKNER R L. Electrochemical methods fundamentals and applications [M]. 2nd ed. New York: John Wiley & Sons, Inc., 2001.
[28] ZHU Xiao-lin, XU Cun-ying, TANG Jie, HUA Yi-xin, ZHANG Qi-bo, LIU Hai, WANG Xiang, HUANG Meng-ting. Selective recovery of zinc from zinc oxide dust using choline chloride based deep eutectic solvents [J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 2222-2228.
[29] YANG H X, REDDY R G. Fundamental studies on electrochemical deposition of lead from lead oxide in 2:1 urea/choline chloride ionic liquids [J]. Journal of the Electrochemical Society, 2014, 161: D586-D592.
[30] KERREC O, DEVILLIERS D, GROULT H, MARCUS P. Study of dry and electrogenerated Ta2O5 and Ta/Ta2O5/Pt structures by XPS [J]. Materials Science and Engineering B, 1998, 55: 134-142.
[31] LIU Peng, DU Yu-ping, YANG Qi-qin, TONG Ye-xiang, HOPE G A. Induced codeposition of Sm-Co amorphous films in urea melt and their magnetism [J]. Journal of the Electrochemical Society, 2006, 153: C57-C62.
[32] YANG Ying-ya, XU Cun-ying, HUA Yi-xin, WANG Meng-meng, SU Zhao-lei. Electrochemical preparation of Ni-La alloys from the EMIC-EG eutectic-based ionic liquid [J]. Ionics, 2017, 23: 1703-1710.
刘爱民1,郭梦霞1,吕梓阳1,张保国1,刘风国1,陶文举1,杨酉坚1,胡宪伟1,王兆文1,刘玉宝2,石忠宁3
1. 东北大学 多金属共生矿生态化冶金教育部重点实验室,沈阳 110819;
2. 包头稀土研究院 白云鄂博稀土资源研究与综合利用国家重点实验室,包头 014030;
3. 东北大学 轧制技术及连轧自动化国家重点实验室,沈阳 110819
摘 要:以钨丝为工作电极,通过循环伏安法研究碳酸乙烯酯-氯化铝(EC-AlCl3)溶剂化离子液体中Ta(V)的电化学还原机理。在EC-AlCl3-TaCl5离子液体的循环伏安曲线中出现4个还原峰,其中-0.55、-0.72和-1.12 V(vs Al)处还原峰对应的反应为Ta(V)络合离子三步还原为金属钽,包括Ta(IV)和Ta(III)络合离子的形成。络合离子Ta(III)被还原为金属钽的过程受扩散控制且不可逆,其在323 K下的扩散系数为3.7×10-7 cm2/s,扩散活化能为77 kJ/mol。另外,对323 K下的阴极产物进行扫描电子显微镜、能量分散光谱和X射线光电子能谱分析。结果表明,在-0.8 V下恒电位电沉积2 h的产物为金属钽和钽的氧化物。
关键词:电化学行为;离子液体;循环伏安法;氯化铝;钽
(Edited by Wei-ping CHEN)
Foundation item: Projects (N182503033, N172502003) supported by the Fundamental Research Funds for the Central Universities, China; Project (2018M640258) supported by Postdoctoral Research Foundation of China; Project (GUIKE AA18118030) supported by Guangxi Innovation-driven Development Program, China
Corresponding author: Zhong-ning SHI; Tel: +86-24-83686381; E-mail: znshi@mail.neu.edu.cn
DOI: 10.1016/S1003-6326(20)65379-1

