高锰酸钾氧化嗅味物质β-环柠檬醛的动力学
张可佳,高乃云,黎雷
(同济大学 污染控制与资源化研究国家重点实验室,上海 200092)
摘要:采用水处理常用氧化剂高锰酸钾对铜绿微囊藻产生的主要致嗅物质β-环柠檬醛(β-cyclocitral)进行氧化去除和动力学分析,并研究高锰酸钾对铜绿微囊藻细胞的破坏和β-环柠檬醛释出及其降解情况。研究结果表明:高锰酸钾与β-环柠檬醛反应符合准二级动力学模型,在溶液pH=7和温度15 ℃时其速率常数为107.2 mol-1·L·s-1;溶液pH对反应影响不大;当pH=2~9时,动力学表观速率常数kobs为(0.078±0.016) min-1;高锰酸钾的加入造成藻细胞的破裂程度达到90%,使主要存在于胞内的β-环柠檬醛释出并降解;高锰酸钾能将铜绿微囊藻细胞内的β-胡萝卜素氧化成β-环柠檬醛,导致总β-环柠檬醛浓度在氧化过程中先升高后降低。
关键词:β-环柠檬醛;高锰酸钾;铜绿微囊藻;β-胡萝卜素;动力学
中图分类号:X524 文献标志码:A 文章编号:1672-7207(2011)04-1161-06
Kinetics of oxidation of odorant β-cyclocitral
by potassium permanganate
ZHANG Ke-jia, GAO Nai-yun, LI Lei
(State Key Laboratory of Pollution Control and Resource Reuse, Tongji University, Shanghai 200092, China)
Abstract: The degradation and its kinetics of typical odorant β-cyclocitral of Microcystis aeruginosa by potassium permanganate were studied. In addition, the effect of oxidation on cell integrity and the release and degradation of β-cyclocitral were investigated. The results indicate that the reaction of potassium permanganate with β-cyclocitral follows the quasi secondary kinetics. The rate constant at pH=7 and 15 ℃ is 107.2 mol-1·L·s-1. The influence of pH on the process is not appreciable and the observed rate constant remains (0.078±0.016) min-1 with pH=2-9. After the oxidation of Microcystis-laden water, about 90% cells are damaged and β-cyclocitral is released to the outside of the cell. The oxidative cleavage of β-carotene by potassium permanganate leads to the formation of β-cyclocitral, which results in the total concentration of β-cyclocitral increase firstly and then decrease during the process.
Key words: β-cyclocitral; potassium permanganate; Microcystis aeruginosa; β-carotene; kinetics
饮用水中的嗅味问题在世界许多国家常是民众抱怨水质不佳的主要原因。美国自来水协会的调查结果表明[1],在388个受访的水厂中,有43%的水厂存在过持续时间超过1周的嗅味问题。自来水嗅味的主要来源之一是地表水中蓝绿藻的生长代谢物,其中包括蓝绿藻中由铜绿微囊藻产生的主要嗅味物质β-环柠檬醛(β-cyclocitral)[2-3]。在我国,由β-环柠檬醛引起的水体嗅味逐渐被关注。李大鹏等[4]采用固相微萃取-气相色谱分析法对我国某水库原水进行分析,发现水中存在大量的β-环柠檬醛。2007年无锡市蓝藻爆发事件中,β-环柠檬醛也是主要的致嗅原因之一[5]。β-环柠檬醛属木头味化合物[6],在不同质量浓度下(0.5~80 μg/L)分别为青草味、甘草味、木头味和烟草味。目前,国内对β-环柠檬醛的研究还停留在对水样的调查分析阶段[4-5],对β-环柠檬醛的产生原因、去除方法和效果尚不明确。国外学者主要针对水库嗅味物质进行调查以及研究嗅味产生与藻类生长情况的相关性[7-8];对β-环柠檬醛的去除方法仅局限于臭氧氧化[9],去除效果较好但处理成本较高。本研究采用高锰酸钾对水体中β-环柠檬醛进行了降解动力学研究,采用GC/MS对氧化过程中的β-环柠檬醛进行定量分析,建立了反应速率模型,考察了高锰钾投加量、β-环柠檬醛的不同浓度及溶液pH对降解速率的影响。同时,将高锰酸钾投加到实际高藻水中,考察β-环柠檬醛从藻细胞中释出和降解的程度,得出β-环柠檬醛与DOC的相关性,提出通过检测β-环柠檬醛浓度来衡量藻细胞破坏程度的可行性。
1 材料与方法
1.1 试验药剂与材料
β-环柠檬醛(纯度为90%)购自Adrich,用甲醇配制成质量浓度为100 mg/L的储备液。内标物为1-氯辛烷(纯度99%),购自Flucka。萃取剂正己烷(纯度为99%)购自Sigma。高锰酸钾为分析纯,用Milli-Q 水配制成质量浓度为1 g/L的储备液。缓冲溶液用K2HPO4和KH2PO4配成,以确保反应过程中pH为中性。其他药剂(HCl,NaOH,NaS2O3和 NaCl)均为分析纯,用Milli-Q水配制。铜绿微囊藻藻种Microcystis Aeruginosa(905)购自中科院水生生物研究所,培养基BG11,光照度为2 000 lux。玻璃纤维滤纸(GF50)购自Advantec,孔径为0.5 μm。
1.2 试验方法
用去离子水将β-环柠檬醛和高锰酸钾的储备液稀释成一定浓度,加入缓冲溶液,用磁力搅拌装置使其混合均匀。反应溶液温度为(15±1) ℃,pH=7。在不同时刻取样,用NaS2O3(浓度为0.1 mol/L)中和剩余的高锰酸钾。取样量为5 mL,加入1 g烘干的 NaCl以便于液液萃取。加入1 mL萃取剂正己烷和1 μL质量浓度为500 mg/L的内标物1-氯辛烷,振荡5 min,静置3 min,取上层有机相测定β-环柠檬醛的浓度。
对数生长期的铜绿微囊藻(2.6×1010个/L)与一定浓度的高锰酸钾反应,于不同时刻取样。一部分样品于-20 ℃冰冻4 h以上,再室温解冻,反复3次,然后,离心过膜用于测定总β-环柠檬醛和总DOC(质量分数);另一部分样品采用玻璃纤维滤纸过滤藻细胞,用于测定细胞外的β-环柠檬醛和DOC。
1.3 分析方法
β-环柠檬醛浓度采用QP2010S气相色谱质谱仪(型号为GC/MS,岛津,日本生产)、RTX-5MS毛细管柱(30 m×0.25 mm ID×0.25 μm)测量。升温程序为在40 ℃保温5 min,然后以10 ℃/min的升温速率升温至100 ℃,再以30 ℃/min的速率升温到250 ℃。进样口温度为180 ℃,离子源温度为200 ℃,接口温度为250 ℃,无分流进样模式,柱流压力为90 kPa。选择离子检测模式,β-环柠檬醛的特征离子为67和109,保留时间为13.658 min。内标法定量,内标物1-氯辛烷的特征离子为55和91,保留时间为11.875 min。
DOC采用TOC-VCPH(岛津,日本生产)进行测定。pH采用雷磁pHS-3C精密pH计测定。
2 结果与讨论
2.1 高锰酸钾氧化β-环柠檬醛的动力学研究
当β-环柠檬醛初始质量浓度为500 μg/L时,研究不同高锰酸钾投加量(1.5,3.0,5.0,8.0和10.0 mg/L)对β-环柠檬醛降解的影响,结果如图1所示。
当高锰酸钾投加量为3.0 mg/L时,研究不同初始质量浓度(500,250和100 μg/L) β-环柠檬醛的降解情况,结果如图2所示。
由图1和图2可见:高锰酸钾能较好地氧化β-环柠檬醛,在30 min内均能达到90%的去除率;特别是当高锰酸钾投加量到10 mg/L时,能在10 min内将500 μg/L β-环柠檬醛的去除率达到95%以上。而Dietrich等[10]的研究表明:高锰酸钾无法有效去除β-环柠檬醛,也无法改变其嗅味特性。这主要是与其使用的测定方法即嗅味层析法(FPA)不稳定,且易受干扰有关。
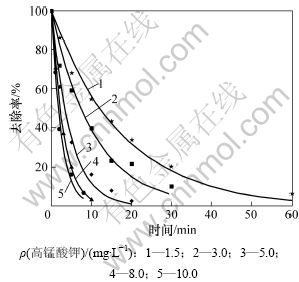
图1 不同高锰酸钾投加量对β-环柠檬醛降解的影响
Fig.1 Effect of various KMnO4 dosages on degradation of β-cyclocitral
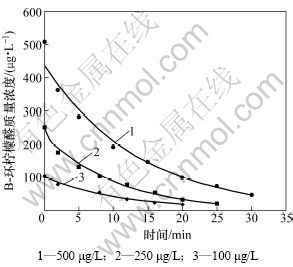
图2 不同初始浓度β-环柠檬醛的降解
Fig.2 Degradation of β-cyclocitral with different initial concentrations
有研究认为高锰酸钾与一些有机物反应,如三氯乙烯[11]、四氯乙烯[12]、叔丁基甲基醚[13]和藻毒素MC-RR[14],都符合准二级动力学反应模型,由此假设高锰酸钾与β-环柠檬醛反应也符合此规律,如式(1)所示:
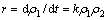 (1)
(1)
式中:k为反应速率常数;ρ1和ρ2分别为β-环柠檬醛与高锰酸钾的质量浓度。
当ρ2>>ρ1,且在试验过程中,高锰酸钾的剩余量几乎没有消耗时,ρ2可作为定值,式(1)可以转化为式(2)和(3):
 (2)
(2)
 (3)
(3)
其中,kobs是关于β-环柠檬醛的准一级动力学表观速率常数;ρ2,0是高锰酸钾的初始质量浓度。
由图1和图2可见:实验所得的数据点能很好地拟合式(2),其相关系数R2>0.97。通过不同质量浓度的β-环柠檬醛或高锰酸钾可以得到不同的kobs,如表1所示。可见:β-环柠檬醛被高锰酸钾降解的速率与β-环柠檬醛的初始浓度无关;而随着高锰酸钾投加量的增加而提高。图3所示为高锰酸钾的投加量与表观速率常数的关系。由此可见:它们符合很好的线性关系(R2>0.99)。经计算得到反应速率常数为107.2 mol-1·L?s-1。由此说明假设是正确的,高锰酸钾氧化β-环柠檬醛符合二级动力学模型,可由式(4)表示:
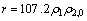 (4)
(4)
表1 β-环柠檬醛与高锰酸钾反应的实验条件和动力学表观速率常数
Table 1 Experimental conditions and kinetics observed rate constants for reaction of β-cyclocitral and KMnO4 (pH=7, t=15 ℃)

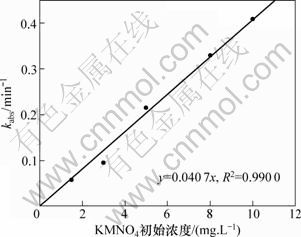
图3 准一级动力学表观速率常数kabs与高锰酸钾投加量的关系
Fig.3 Relationship between pseudo first-order observed rate constant and dosage of KMnO4
高锰酸钾氧化β-环柠檬醛的速率常数为107.2 mol-1?L?s-1,比氧化三氯乙烯的速率常数0.067 mol-1?L?s-1[11]高,比氧化MC-RR的速率常数469 mol-1?L?s-1[14]和MC-LR的速率常数357.2 mol-1·L?s-1[15]低。由此说明:高锰酸钾氧化有机物具有一定的选择性,同时,高锰酸钾虽然对于β-环柠檬醛氧化效果明显,但与藻类的其他代谢物(如藻毒素MC-LR,MC-RR)相比,氧化速率更低,并非之前认为的嗅味物质容易去除。因此,关于藻类的嗅味代谢物β-环柠檬醛的去除方法的研究应得到进一步加强。
2.2 不同pH对反应的影响
溶液的酸碱性会影响高锰酸钾的氧化还原电 位[16],因此,pH是研究高锰酸钾氧化试验的重要参数。Yan等[17]认为:在强酸条件下(pH<3.5),氧化还原电位Eo=+1.51 V,反应按式(5)进行。在pH为3.5~12时,氧化还原电位较低,MnO4-主要被还原成MnO2,反应按式(6)和(7)进行。Aleboyeh等[16]采用高锰酸钾氧化偶氮基染料时发现:氧化效果随反应pH的下降而增强。可见,酸性条件有助于反应的进行。
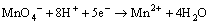 (5)
(5)
 (6)
(6)
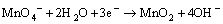 (7)
(7)
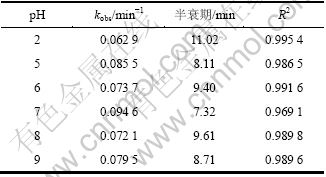
为考察不同pH对β-环柠檬醛去除率的影响,加入HCl或NaOH调节反应溶液的pH至2,5,6,8和9。高锰酸钾投加量为3.0 mg/L,β-环柠檬醛初始质量浓度为500 μg/L。不同pH条件下的表观速率常数kobs和半衰期如表2所示。结果表明,当pH=2~9时,表观速率常数kobs变化不大。Chen等[14]在氧化MC-RR时发现:当pH从5升高到9时,降解速率由1.176 min-1降低至0.919 min-1;Yan等[11]在研究高锰酸钾氧化三氯乙烯也发现,pH为4~8时,反应速率常数保持在(0.67±0.03) mol-1?L?s-1内。这些结论与本节开始讨论的理论相悖,由此推测,氧化过程除受pH影响外,可能还与反应物质的结构有关[16]。
表2 不同pH条件下高锰酸钾降解β-环柠檬醛的动力学模型的拟合参数
Table 2 Fitting parameters of kinetics models on degradation of β-cyclocitral by KMnO4 under different pH
2.3 铜绿微囊藻中β-环柠檬醛的释出及其降解
为研究高锰酸钾对铜绿微囊藻细胞内β-环柠檬醛释出及其降解情况,配制藻细胞浓度为2.6×1010个/L,高锰酸钾质量浓度为20 mg/L。藻细胞中β-环柠檬醛和DOC释出及其降解如图4所示。从图4可知:在20 mg/L 高锰酸钾的作用下,总DOC随时间变化不明显,说明高锰酸钾对藻类代谢的有机物的碳化能力不强,这与前人结论一致[18-19]。反应60 min后,胞外的DOC由16.65 mg/L增加到42.60 mg/L,占总DOC的90%~93%。可见:当高锰酸钾氧化到60 min时,藻细胞基本全部破裂,藻内的代谢物释出。在0 min时,胞外的β-环柠檬醛检测不到,说明β-环柠檬醛主要存在于藻细胞内。反应至60 min时,胞外的β-环柠檬醛质量浓度也突然增加,且其上升趋势与胞外的DOC上升趋势相似,推测β-环柠檬醛与DOC相关,可作为藻细胞内部代谢物的代表物。通过测定β-环柠檬醛的质量浓度可看出胞内代谢物的释出情况,从而了解藻细胞的破裂程度。
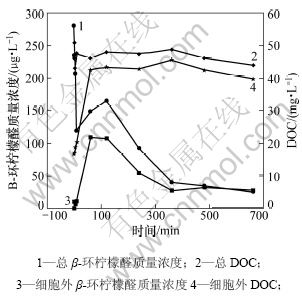
图4 高锰酸钾质量浓度为20 mg/L时藻细胞中β-环柠檬醛和DOC释出及其降解
Fig.4 Release and degradation of β-cyclocitral and DOC from microcystis aeruginosa when KMnO4 concentration is 20 mg/L
不同质量浓度(20和40 mg/L)的高锰酸钾对β-环柠檬醛降解的影响如图5所示。可见:随着高锰酸钾投加量的增加,氧化性能增强,40 mg/L 高锰酸钾能在4 h内将总的β-环柠檬醛降解到检测不到。在反应2 min后,胞内的β-环柠檬醛就开始明显释出;10 min后β-环柠檬醛的质量浓度达到80.99 μg/L。在氧化反应前期(0~5 min),总的β-环柠檬醛受高锰酸钾氧化后质量浓度降低,这说明高锰酸钾能有效地降解β-环柠檬醛。在氧化5 min后,总β-环柠檬醛质量浓度开始增加,当高锰酸钾投加量为20 mg/L时,在120 min后,β-环柠檬醛的质量浓度达到新的峰值165.33 μg/L;当高锰酸钾投加量为40 mg/L时,在30 min后β-环柠檬醛的质量浓度达到新的峰值180.02 μg/L。随着反应时间的延长,β-环柠檬醛质量浓度再次降低。出现总β-环柠檬醛质量浓度上升的现象可能是在氧化过程中又产生了新的β-环柠檬醛。Jüttner等[20]在1985年已经证实β-环柠檬醛是微囊藻的主要挥发性代谢物,微囊藻胞内的β-胡萝卜素(β-carotene)在β-胡萝卜素加氧酶(β-carotene oxygenase)的催化下被氧化成β-环柠檬醛,其反应如图6所示。同时,β-胡萝卜素化学性质不稳定,易在光照和加热时发生氧化反应。李明洁[21]认为:β-胡萝卜素会被臭氧氧化,从而使β-环柠檬醛的质量浓度在反应过程中先升高后下降。这可能是藻内剩余的β-胡萝卜素也会被高锰酸钾氧化,生成新的β-环柠檬醛,造成质量浓度升高。
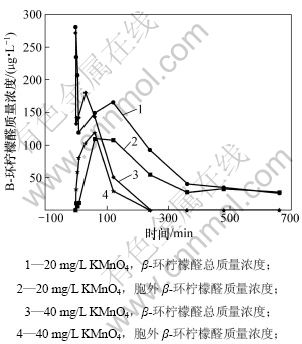
图5 不同投加量高锰酸钾对β-环柠檬醛降解的影响
Fig.5 Effect of various KMnO4 dosages on degradation of β-cyclocitral

图6 β-胡萝卜素的裂解反应
Fig.6 Cleavage reaction of β-carotene
3 结论
(1) 高锰酸钾能较好地氧化β-环柠檬醛,在30 min内均能达到90%的去除率。高锰酸钾与β-环柠檬醛的反应符合准二级动力学反应模型,其速率常数为107.2 mol-1?L?s-1,其动力学方程式为r=107.2ρ1ρ2,0。
(2) 在pH=2~9范围内,高锰酸钾降解β-环柠檬醛的速率常数kobs为(0.078±0.016) min-1,可以忽略pH的变化对降解效果造成的影响。
(3) β-环柠檬醛主要存在于藻细胞内,高锰酸钾的加入会造成藻细胞的破裂,从而使藻体代谢物包括β-环柠檬醛释出。胞外β-环柠檬醛浓度的变化与释出的DOC有较好的相关性。
(4) 高锰酸钾能将藻细胞内的β-胡萝卜素氧化成β-环柠檬醛,β-环柠檬醛的生成和降解速率会根据高锰酸钾的投加量达到平衡,其浓度在反应过程中出现先升高再降低的现象。
参考文献:
[1] Suffet I H, Corado A, Chou D, et al. AWWA taste and odor survey[J]. Journal of the American Water Works Association, 1996, 88(4): 168-180.
[2] Slater G P, Block V C. Volatile compounds of the cyanophyceae-a review[J]. Water Science and Technology, 1983, 15(6/7): 181-190.
[3] Suffet I H, Khiari D, Bruchet A. The drinking water taste and odor wheel for the millennium: Beyond geosmin and 2-methylisoborneol[J]. Water Science and Technology, 1999, 40(6): 1-13.
[4] 李大鹏, 李伟光. S市水源水致臭物质分析研究[J]. 苏州科技学院学报: 工程技术版, 2006, 19(2): 52-53, 66.
LI Da-peng, LI Wei-guang. Analysis of moderately volatile taste and odor compounds from raw water in S city[J]. Journal of University of Science and Technology of Suzhou: Engineering and Technology, 2006, 19(2): 52-53, 66.
[5] 于建伟, 李宗来, 曹楠, 等. 无锡市饮用水嗅味突发事件致嗅原因及潜在问题分析[J]. 环境科学学报, 2007, 27(11): 1771-1777.
YU Jian-wei, LI Zong-lai, CAO Nan, et al. Analyses on cause for odor and potential problems in water source during odor episode event in Wuxi[J]. Acta Scientiae Circumstantiae, 2007, 27(11):1771-1777.
[6] Young C C, Suffet I H, Crozes G, et al. Identification of a woody-hay odor-causing compound in a drinking water supply[J]. Water Science and Technology, 1999, 40(6): 273-278.
[7] Ikawa M, Sasner J J, Haney J F. Activity of cyanobacterial and algal odor compounds found in lake waters on green alga Chlorella pyrenoidosa growth[J]. Hydrobiologia, 2001, 443(1/3): 19-22.
[8] Peter A, Koster O, Schildknecht A, et al. Occurrence of dissolved and particle-bound taste and odor compounds in Swiss lake waters[J]. Water Research, 2009, 43(8): 2191-2200.
[9] Peter A, Von G U. Oxidation kinetics of selected taste and odor compounds during ozonation of drinking water[J]. Environmental Science and Technology, 2007, 41(2): 626-631.
[10] Dietrich A M, Hoehn R C, Dufresne L C, et al. Oxidation of odorous and nonodorous algal metabolites by permanganate, chlorine and chlorine dioxide[J]. Water Science Technology, 1995, 31(11): 223-228.
[11] Yan Y E, Schwartz F W. Kinetics and mechanisms for TCE oxidation by permanganate[J]. Environmental Science and Technology, 2000, 34(12): 2535-2541.
[12] Huang K C, Hoag G E, Chheda P, et al. Kinetics and mechanism of oxidation of tetrachloroethylene with permanganate[J]. Chemosphere, 2002, 46(6): 815-825.
[13] Damm J H, Hardacre C, Kalin R M, et al. Kinetics of the oxidation of methyl tert-butyl ether (MTBE) by potassium permangnate[J]. Water Research, 2002, 36(14).
[14] Chen X G, Xiao B D, Liu J T, et al. Kinetics of the oxidation of MCRR by potassium permangante[J]. Toxicon, 2005, 45(7): 911-917.
[15] Rodríguez E, Majado M E, Meriluoto J, et al. Oxidreation of microcystins by permanganate: reaction kinetics and implications for water treatment[J]. Water Research, 2007, 41(1): 102-110.
[16] Aleboyeh A, Olya M E, Aleboyeh H. Oxidative treatment of azo dyes in aqueous solution by potassium permangante[J]. Journal of Hazardous Materials, 2008, 162(2-3): 1530-1535.
[17] Yan Y E, Schwartz F W. Oxidation degradation and kinetics of chlorinated ethylenes by potassium permanganate[J]. Journal of Contaminant Hydrology, 1999, 37(3/4): 343-365.
[18] Guo Z H, Pei Y S, Yang M, et al. Removal of organics and control of bromate for a southern China water supply[J]. Journal American Water Works Association, 2007, 99(10): 110-116.
[19] Skjemstad J O, Swift R S, McGowan J. Comparison of particulate organic carbon and permanganate oxidation methods for estimating labile soil organic carbon[J]. Australian Journal of Soil Reasearch, 2006, 44(3): 255-263.
[20] Jüttner F, Hiiflacher B. Evidence of β-carotene 7,8 (7',8') oxygenase (β-cyclocitral,crocetindial generating) in microcystis[J]. Archives of Microbiology, 1985, 141(4): 337-343.
[21] 李明洁. 臭氧对两种产臭蓝绿菌菌体破坏及其代谢物释出之研究[D]. 台南: 国立成功大学环境工程系, 2008: 50-63.
LI Ming-jie. Effect of ozonation on two nauseous cyanobacteria cells and release of their metabolites[D]. Tainan: National Cheng Kung University. Department of Environmental Engineering, 2008: 50-63.
(编辑 赵俊)
收稿日期:2010-01-29;修回日期:2010-04-17
基金项目:国家科技重大专项资助项目(2008ZX07421-002;2008ZX07421-004);高技术研究发展计划(“863”计划)项目(2008AA06A412)
通信作者:高乃云(1950-),女,陕西府谷人,博士,教授,博士生导师,从事饮用水处理技术;电话:021-65982691;E-mail:gaonaiyun@sina.com