DOI: 10.11817/j.issn.1672-7207.2016.11.003
金属热还原法制备V-Ti基合金的动力学研究
王斌1,杜金晶1,刘奎仁2,朱军1,李林波1
(1. 西安建筑科技大学 冶金工程学院,陕西 西安,710055;
2. 东北大学 材料与冶金学院,辽宁 沈阳,110819)
摘要:采用DSC测试技术对金属热还原不同反应体系的放热过程进行研究,并采用Freeman-Carroll法,对相关反应的动力学参数进行计算。研究结果表明:Al与V2O5的反应主要为液-液反应,在1 000 ℃以下就可进行;Al与TiO2可以以液-固形式反应,但在1 100 ℃以上时,反应才显著发生;以Al-Ca合金替代Al粉作为还原剂,可以促进热还原反应提前发生,但钙热反应先于铝热反应出现;Al与TiO2反应的活化能明显高于Al与V2O5反应的活化能,反应速率受温度的影响更大;原料中添加一定量CaO,可以促进Al与TiO2反应的发生,降低此反应的活化能,但会增加Al与V2O5反应的活化能。
关键词:V-Ti基合金;金属热还原法;动力学
中图分类号:TF803.13 文献标志码:A 文章编号:1672-7207(2016)11-3635-07
Kinetic study on preparation of V-Ti based alloys by metallothermic reduction method
WANG Bin1, DU Jinjing1, LIU Kuiren2, ZHU Jun1, LI Linbo1
(1. School of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China;
2. School of Materials & Metallurgy, Northeastern University, Shenyang 110819, China)
Abstract: The DSC curves were tested to study the exothermic processes of different reaction systems, and the kinetic parameters of the relevant reactions were calculated using Freeman-Carroll method. The results show that Al(liquid) reacts with V2O5(liquid) below 1 000 ℃. However Al(liquid)-TiO2(solid) reaction occurs obviously only when the temperature is higher than 1 100 ℃. If Al-Ca alloy is used as the reducing agent, the thermal reactions appear in advance, and the calcium thermal reductions occur earlier than the thermite reactions. The apparent activation energy of the Al-TiO2 reactions are notably larger than that of Al-V2O5 reactions, and the reaction rates of Al-TiO2 reactions are more sensitive to the temperature. Adding some CaO to Al-TiO2-V2O5 reaction system can reduce the apparent activation energy of the Al-TiO2 reaction, but it can also increase the apparent activation energy of the Al-V2O5 reaction.
Key words: V-Ti based alloy; metallothermic reduction method; kinetic
钒钛基固溶体储氢合金具有理论储氢量高、易活化、抗粉化性能好和可在常温下实现吸放氢等优点,被认为是一种具有良好应用前景的储氢材料[1]。在V-Ti基固溶体储氢合金体系中,目前研究较多的主要有Ti-V二元系[2],Ti-V-Cr,Ti-V-Mn,Ti-V-Fe和Ti-V-Ni等三元系[3-5],Ti-V-Cr-Mn,Ti-V-Cr-Fe和Ti-V-Cr-Zr等四元系及其他多元系[6-7]。其制备方法普遍采用兑掺法,即以钒、钛等纯金属为原料,利用感应炉或电弧炉反复熔炼获得。因为纯钒价格昂贵,加之钒、钛等金属熔点过高,导致了合金的制备成本过高,限制了它的规模开发利用[8]。为降低其制备成本,目前有研究以金属热还原法进行合金的制备[9-13],即以价格较低廉的钒、钛等金属氧化物为原料,通过自放热反应进行合成。该反应过程具有高温和快速的特点,这一特点在一定程度上可以起到促进杂质元素挥发和提升生产速率的作用,但另一方面也增加了对其反应过程研究的难度。目前,关于各反应放热情况及其相关的动力学过程研究,还少有报道。本文作者采用DSC测试技术对金属热还原法制备V-Ti基合金的动力学过程进行了研究,并采用Freeman-Carroll法计算了还原过程中各反应的表观活化能、反应级数和频率因子等动力学参数。
1 实验
本文采用非等温法研究不同反应体系的放热过程,即在线性升温条件下测出DSC曲线,再利用DSC曲线求出相关反应的动力学参数。典型的DSC曲线如图1所示,设反应初始温度T0到反应结束温度T2的DSC曲线总面积为S,T0→T1的曲线面积为S1,T1→T2的曲线面积为S2。根据Freeman-Carroll微分数据处理方法,图1中各参数存在如下等式关系:
 。因此,对于1条DSC曲线来说,只要求出曲线上一些离散点对应的△Q,T和S2,便可作出
。因此,对于1条DSC曲线来说,只要求出曲线上一些离散点对应的△Q,T和S2,便可作出 关系曲线,进而由直线斜率算得活化能E,由直线截距得到反应级数n,最后代入E和n求出反应频率因子和速率常数[14–15]。
关系曲线,进而由直线斜率算得活化能E,由直线截距得到反应级数n,最后代入E和n求出反应频率因子和速率常数[14–15]。

图1 DSC曲线示意图
Fig. 1 Schematic diagram of DSC curve
测试的反应体系包括Al-TiO2体系、Al-V2O5体系、Al-TiO2-V2O5体系、Al-TiO2-V2O5-CaO体系和(Al-Ca合金)-TiO2-V2O5-CaO体系。试剂主要有五氧化二钒、二氧化钛、铝钙合金、铝粉、氧化钙等,其中铝钙合金成分为Al(20%)-Ca(80%),杂质质量分数小于0.3%,其余均为分析纯。测试前,各原料均在200 ℃下烘干3 h(铝粉、铝钙合金除外),以去除其中水分。另外,为保证物料混合均匀,所有原料均经充分研磨,粒度小于50 μm。
采用德国NETZSCH公司生产的STA 409C/CD型同步热分析仪进行测试,样品加入量5 mg,升温速率为10 ℃/min,最高温度为1 400 ℃,氩气流量为50 mL/min。
2 结果与讨论
2.1 Al-TiO2体系
图2所示为Al-TiO2体系的DSC曲线。由图2可以看出:曲线在658 ℃出现第1个峰值,为Al熔化的吸热峰。温度升高到1 212 ℃时开始出现第2个峰,为Al(液态)与TiO2(固态)反应的放热峰。

图2 Al-TiO2体系的DSC曲线
Fig. 2 DSC curve of Al-TiO2 system
对Al-TiO2体系的放热峰进行数据分析,绘制 曲线,如图3所示。
曲线,如图3所示。

图3 Al与TiO2反应的Freeman-Carroll分析
Fig. 3 Freeman-Carroll analysis of reaction between Al and TiO2
拟合的方程为y=-192 388x+0.86,由此计算Al与TiO2反应的表观活化能E=1 599.5 kJ/mol,反应级数n=0.86,频率因子A=3.7×1053 s-1,动力学方程为: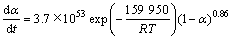 。
。
2.2 Al-V2O5体系
图4所示为Al-V2O5体系的DSC曲线。曲线在658 ℃和698 ℃出现了2个吸热峰,分别代表着Al和V2O5的熔化。曲线第3个峰为放热峰,在801 ℃时出现,为Al(液态)与V2O5(液态)反应的放热峰。

图4 Al-V2O5体系的DSC曲线
Fig. 4 DSC curve of Al-V2O5 system
对Al-V2O5体系的放热峰进行数据分析,绘制 曲线,如图5所示。
曲线,如图5所示。

图5 Al与V2O5反应的Freeman-Carroll分析
Fig. 5 Freeman-Carroll analysis of reaction between Al and V2O5
拟合的方程为y=-45 877.1x+0.66,由此计算Al与V2O5反应的表观活化能E=381.4 kJ/mol,比前面Al与TiO2反应的活化能要小。由于活化能在一定程度上可以反映某反应进行的难易程度,因此,可以确定Al与V2O5的反应更容易进行,反应速率较大,并且受温度的影响较小。
由图5拟合的方程可知:反应级数n=0.66,频率因子A=2.3×1016 s-1,反应的动力学方程为:
 。
。
2.3 Al-TiO2-V2O5体系
图6所示为Al-TiO2-V2O5体系的DSC曲线。由 图6可以看出:曲线在658 ℃和690 ℃出现了2个吸热峰,分别为Al和V2O5的熔化峰。当温度升高到961 ℃,时出现第1个放热峰,表示Al(液态)与V2O5(液态)的反应发生;继续升温到1 318 ℃,出现了第2个放热峰,为Al(液态)与TiO2(固态)反应的放热峰。与图2和图4进行对比可知:以TiO2和V2O5的混合物料作为反应物时,Al热反应发生的起始温度均有所升高,这主要与混合物料的相界面更复杂有关。

图6 Al-TiO2-V2O5体系的DSC曲线
Fig. 6 DSC curve of Al-TiO2-V2O5 system
对Al-TiO2-V2O5体系的2个放热峰进行数据分析,绘制Al与TiO2反应、Al与V2O5反应的 曲线,如图7和图8所示。
曲线,如图7和图8所示。
Al与V2O5反应拟合的方程为y=-34 050x+0.49,由此计算反应的表观活化能E=283.1 kJ/mol,小于Al与V2O5单独反应时的活化能(381.4 kJ/mol)。这主要是因为Al与V2O5之间的反应为液-液反应,升高反应温度,增加了反应物原子之间的有效碰撞,从而降低了反应进行的难度。

图7 Al与V2O5反应的Freeman-Carroll分析 (Al-TiO2-V2O5体系)
Fig. 7 Freeman-Carroll analysis of reaction between Al and V2O5 for Al-TiO2-V2O5 system

图8 Al与TiO2反应的Freeman-Carroll分析 (Al-TiO2-V2O5体系)
Fig. 8 Freeman-Carroll analysis of reaction between Al and TiO2 for Al-TiO2-V2O5 system
由图7拟合的方程可知:反应级数n=0.49,频率因子A=3.9×109s-1,动力学方程为:
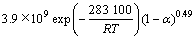 。
。
Al与TiO2反应拟合的方程为y=-579 341x+2.03,由此计算反应的表观活化能E=4 816.6 kJ/mol,明显大于Al与TiO2单独反应时的活化能(1 599.5 kJ/mol)。这很可能是因为Al与V2O5反应过后,反应产物覆盖在了TiO2颗粒的表面,从而进一步增加了反应进行的难度。
由图8拟合的方程可知:反应级数n=2.03,频率因子A=1.3×10156 s-1,动力学方程为:
 。
。
2.4 Al-TiO2-V2O5-CaO体系
图9所示为Al-TiO2-V2O5-CaO体系的DSC-TG曲线。由图9可知:因为CaO的加入,曲线在383 ℃就出现了第1个吸热峰,表示Ca(OH)2脱水。Ca(OH)2的形成主要是由于CaO极易吸水,其中不可避免地会存在一些水解产物。曲线在658 ℃和688 ℃出现了2个吸热峰,分别为Al和V2O5的熔化峰。当温度升高到920 ℃和1 115 ℃时,出现了2个放热峰。分别对应Al(液态)与V2O5(液态)的反应、Al(液态)与TiO2(固态)的反应。与图6进行对比可知:Al与TiO2反应的放热峰出现时间明显提前,这说明加入一定的CaO可以促进此反应发生。

图9 Al-TiO2-V2O5-CaO体系的DSC-TG曲线
Fig. 9 DSC-TG curves of Al-TiO2-V2O5-CaO system
对Al-TiO2-V2O5-CaO体系的2个放热峰进行数据分析,绘制Al与TiO2反应、Al与V2O5反应的 曲线,如图10和图11所示。
曲线,如图10和图11所示。

图10 Al与V2O5反应的Freeman-Carroll分析(Al-TiO2-V2O5-CaO体系)
Fig. 10 Freeman-Carroll analysis of reaction between Al and V2O5 for Al-TiO2-V2O5-CaO system

图11 Al与TiO2反应的Freeman-Carroll分析(Al-TiO2-V2O5-CaO体系)
Fig. 11 Freeman-Carroll analysis of reaction between Al and TiO2 for Al-TiO2-V2O5-CaO system
Al与V2O5反应拟合的方程为y=-50 059x+1.11,由此计算Al与V2O5反应的表观活化能E=416.19 kJ/mol,反应级数n=1.11,频率因子A=1.6×1015 s-1,动力学方程为

Al与TiO2反应拟合的方程为y=-334 338x+2.16,由此计算反应的表观活化能E=2 779.6 kJ/mol,反应级数n=2.16,频率因子A=5.3×10103 s-1,动力学方程为: 。
。
与2.3节中数据进行对比可知:添加CaO后,Al与V2O5反应的活化能增加,Al与TO2反应的活化能减小。这说明,添加CaO有利于TiO2还原反应的进行,而不利于V2O5的还原反应。因此,在实际冶炼中,需要控制CaO的加入量,不能过多,否则会影响V2O5的还原反应。
2.5 (Al-Ca)-TiO2-V2O5-CaO体系
图12所示为(Al-Ca)-TiO2-V2O5-CaO体系的DSC曲线。由图12可见:曲线在381 ℃出现了第1个吸热峰,同样为Ca(OH)2的脱水反应峰;542 ℃时,Al-Ca合金熔化,形成了第2个吸热峰;当温度升高到681 ℃左右时,曲线出现波动,V2O5开始熔化;在707 ℃,曲线出现了1个大的放热峰,这一现象在上述几条DSC曲线并未出现,说明此处的放热峰必然与Ca的参与有关,反应为钙热反应。钙热反应放热峰峰型锐利,说明此反应受温度的影响非常大。温度继续升高,曲线在939 ℃出现了1个小的放热峰,通过与前面几条DSC曲线进行对比可知:此处的反应为Al(液态)与V2O5(液态)的反应。与Al-TiO2-V2O5-CaO体系不同,曲线在1 000 ℃以上并没有出现明显的铝热反应放热峰。推测其原因,很可能是钙热反应释放了大量热,导致体系温度的急剧升高,进而诱发了多种反应的同时发生,大部分实验用料提前消耗。
对(Al-Ca)-TiO2-V2O5-CaO体系的2个放热峰进行数据分析,绘制钙热反应和铝热反应的
 曲线,如图13和图14所示。
曲线,如图13和图14所示。

图12 (Al-Ca)-TiO2-V2O5-CaO体系的DSC曲线
Fig. 12 DSC curve of (Al-Ca)-TiO2-V2O5-CaO system

图13 钙热反应Freeman-Carroll分析((Al-Ca)-TiO2-V2O5-CaO体系)
Fig. 13 Freeman-Carroll analysis of calciothermics reactions for (Al-Ca)-TiO2-V2O5-CaO system

图14 铝热反应Freeman-Carroll分析((Al-Ca)-TiO2-V2O5-CaO体系)
Fig. 14 Freeman-Carroll analysis of aluminothermy reduction reactions for (Al-Ca)-TiO2-V2O5-CaO system
钙热反应拟合的方程为y=-368 266x+2.63,由此计算反应的表观活化能E=3 061.8 kJ/mol。活化能较高,很可能是因为反应进行时的温度较低,V2O5还未熔化完全,从而导致整体反应进行困难。反应级数n=2.63,频率因子A=1.9×10161 s-1,动力学方程为: 。
。
铝热反应拟合的方程为y=-46 805x+0.65,由此计算反应的表观活化能E=389.1 kJ/mol,反应级数n=0.65,频率因子A=3.7×1014 s-1,动力学方程为: 。
。
3 结论
1) Al与V2O5的反应主要为液-液反应,在1 000 ℃内就可进行,Al与TiO2可以液-固的形式反应,但在1 100 ℃以上时反应才显著发生。
2) 以Al-Ca合金替代Al粉作为还原剂,可以促进热反应提前发生,但钙热反应先于铝热反应出现。
3) Al-TiO2反应的活化能明显高于Al-V2O5反应的活化能,反应速率受温度的影响更大。原料中添加一定CaO,可以降低Al-TiO2反应的活化能,促进此反应的发生。
参考文献:
[1] 柴玉俊, 赵敏寿. 几种固溶体储氢合金的研究近况[J]. 稀有金属材料与工程, 2005, 34(2): 174-177.
CHAI Yujun, ZHAO Minshou. Recent development of several solid solution hydrogen storage alloys[J]. Rare Metal Materials and Engineering, 2005, 34(2): 174-177.
[2] SUWARNO S, SOLBERG J K, MAEHLEN J P, et al. The effects of rapid solidification on microstructure and hydrogen sorption properties of binary BCC Ti-V alloys[J]. Journal of Alloys and Compounds, 2014, 582(1/2): 540-546.
[3] TOWATA S, NORITAKE T, ITOH A, et al. Effect of partial niobium and iron substitution on short-term cycle durability of hydrogen storage Ti-Cr-V alloys[J]. International Journal of Hydrogen Energy, 2013, 38(7): 3024-3029.
[4] WANG Haibin, WANG Qing, DONG Chuang, et al. Microstructure and storage properties of low V-containing Ti-Cr-V hydrogen storage alloy prepared by arc melting and suction casting[J]. Rare Metals, 2013, 32(4): 354-358.
[5] PICKERING L, LI J, REED D, et al. Ti-V-Mn based metal hydrides for hydrogen storage[J]. Journal of Alloys and Compounds, 2013, 580(Suppl 1): S233-S237.
[6] CHO S, HAN C, PARK C, et al. Hydrogen storage characteristics of Ti-Zr-Cr-V alloys[J]. Journal of Alloys and Compounds, 1999, 289(1/2): 244-250.
[7] DOLAN M D, MCLENNAN K G, SONG G, et al. The effect of Ti on hydrogen absorption and diffusivity in V-Ti-Al alloy membranes[J]. Journal of Membrane Science, 2013, 46(1): 405-409.
[8] 杨绍利. 钒钛材料[M]. 北京: 冶金工业出版社, 2007: 1-10.
YANG Shaoli. Materials of vanadium and titanium[M]. Beijing: Metallurgical Industry Press, 2007: 1-10.
[9] TSUKAHARA M, KAMIYA T. Hydrogen storage and electrode properties of V-based solid solution type alloys prepared by a thermic process[J]. Journal of Electrochemical Society, 2000, 147(8): 2941-2944.
[10] 高田裕章, 冈裕, 中川纯一, 等. Cr-Ti-V系储氢合金的制造方法. 中国, 1522308A[P]. 2002-08-18.
TAKADA H, OKA Y, NAKAGAWA J, et al. The manufacturing method of the Cr-Ti-V hydrogen storage alloy: CN, 1522308A[P]. 2002-08-18.
[11] 李荣, 周上祺, 刘守平, 等. 自蔓延高温合成V3TiNi0.56Al0.2贮氢材料[J]. 重庆大学学报(自然科学版), 2005,28(4): 53-55.
LI Rong, ZHOU Shangqi, LIU Shouping, et al. Materials hydrogen storage V3TiNi0.56Al0.2 by self propagating high temperature synthesis[J]. Journal of Chongqing university (Natural Science Edition), 2005, 28(4): 53-55.
[12] SANTOS S F, HUOT J. Hydrogen storage in TiCr1.2(FeV)x BCC solid solutions[J]. Journal of Alloys and Compounds, 2009, 472(1/2): 247-251.
[13] WANG Bin, LIU Kuiren, CHEN Jianshe, et al. Preparation of V-Ti-Fe master alloys by metallothermic reduction method[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(6): 1507-1512.
[14] 沈兴. 差热、热重分析与非等温固相反应动力学[M]. 北京: 冶金工业出版社, 1995: 100-139.
SHEN Xing. DTA-TG analysis and non-isothermal solid state reaction kinetics[M]. Beijing: Metallurgical Industry Press, 1995: 100-139.
[15] 李余增. 热分析[M]. 北京: 清华大学出版社, 1987: 207-219.
LI Yuzeng. Thermal analysis[M]. Beijing: Tsinghua University Press, 1987: 207-219.
(编辑 陈爱华)
收稿日期:2016-01-07;修回日期:2016-04-05
基金项目(Foundation item):国家自然科学基金资助项目(51404183);西安建筑科技大学人才科技基金资助项目(RC1355) (Project(51404183) supported by the National Natural Science Foundation of China; Project(RC1355) supported by the Fundamental Research Funds of Xi’an University of Architecture and Technology)
通信作者:王斌,博士,讲师,从事稀有金属冶金与应用研究;E-mail: wxauat@126.com