文章编号:1004-0609(2015)-07-1987-06
基于同时平衡原理的Au-I--H2O系热力学分析
李绍英1, 2, 3,赵留成3,孙春宝3,袁喜振3,王培龙3,邓祥意3,刘 柯4
(1. 华北理工大学 矿业工程学院,唐山 063009;
2. 河北省矿业开发与安全技术重点实验室,唐山 063009;
3. 北京科技大学 金属矿山高效开采与安全教育部重点实验室,北京 100083;
4. 中冶华冶(北京) 国际贸易有限公司,北京 100029)
摘 要:采用同时平衡原理对Au-I--H2O系的浸金热力学进行分析,分别研究溶液与固体Au以及AuI沉淀与固体Au平衡时的φ-pH关系、AuI存在条件和溶液中含金组分的变化规律。结果表明:随着溶液中含金组分的总浓度cT(Au)的降低或含碘组分的总浓度cT(I-)的增加,还原电位φsol/Au迅速降低,Au3+/Au2O3的平衡pH值增大,非常有利于金的碘化浸出;随着溶液中cT(I-)逐渐增加,出现AuI沉淀且其沉淀量呈先增大后减小的趋势;在cT(Au)为1×10-4 mol/L的条件下,当溶液中cT(I-)浓度大于0.0021 mol/L时,可避免AuI沉淀的生成,金主要以AuI2-络合离子形式存在。Au-I--H2O系的浸金热力学分析为金的高效碘化浸出提供了理论依据。
关键词:Au-I--H2O系;同时平衡原理;φ-pH图;AuI沉淀;热力学分析
中图分类号:TF111 文献标志码:A
Thermodynamic analysis for Au-I--H2O system based on principle of simultaneous equilibrium
LI Shao-ying1, 2, 3, ZHAO Liu-cheng3, SUN Chun-bao3, YUAN Xi-zhen3,
WANG Pei-long3, DENG Xiang-yi3, LIU Ke4
(1. College of Mining Engineering, North China University of Science and Technology, Tangshan 063009, China;
2. Hebei Province Mining Industry Develops with Safe Technology Priority Laboratory, Tangshan 063009, China;
3. Key Laboratory of the Ministry of Education of China for High-Efficient Mining and Safety of Metal Mines,
University of Science and Technology Beijing, Beijing 100083, China;
4. MCC Huaye (Beijing) International Trade Co., Ltd., Beijing 100029, China)
Abstract: Thermodynamic analysis of Au-I--H2O system was carried out based on the principle of simultaneous equilibrium. The φ-pH programs of balance with solution and Au, solid AuI and Au were investigated. Also, the existence condition of AuI precipitation and change-rule of gold-bearing components were studied. The results show that with decreasing cT(Au) or increasing cT(I-) in the solution, φsol/Au reduces rapidly compared to the standard potential of Au, and the balance pH value of Au3+/Au2O3 increases. This is beneficial to gold leaching with iodine-iodide. As the cT(I-) increases gradually in the solution, AuI precipitation appears and the trend of AuI precipitation amount increases firstly and then reduces. When the cT(Au) is 1×10-4 mol/L, if the cT(I-) is more than 0.0021 mol/L, AuI precipitation will not occur, meanwhile, the main complex ion is AuI2-. The thermodynamic analysis of Au-I--H2O system provides theoretical basis for the efficient gold leaching with iodine-iodide.
Key words: Au-I--H2O system; simultaneous equilibrium principle; φ-pH diagram; AuI precipitation; thermodynamic analysis
目前,世界上主要的提金方法是氰化法。氰化工艺简单,金回收率高,浸出过程容易控制,对矿石适用性强[1];但是它也存在着一些不容忽视的缺点:氰化物有剧毒,环境污染大;浸金速度慢;对细粒包裹金、高砷、高硫、含有机碳的难处理金矿石浸出效果差;浸出过程易受铜、铁、铅、锌和硫等杂质的影响等[2-3]。因此,科研人员一直致力于非氰浸金技术的研究。国内外已有的非氰浸金方法主要有硫脲法、硫代硫酸盐法、石硫合剂法、卤素提金法、丙二腈法(有机腈法)、硫氰化物法、多硫化物法等[4-7]。
碘化法是一种非氰浸金方法,早在20世纪90年代,国外一些研究人员就开始对碘化浸金进行研究,并取得了一定的成果。QI等[8-9]研究了金在碘-碘化物溶液中的动力学及电化学行为,结果表明金的溶解遵循一级反应动力学,一级反应速率与圆盘旋转速度的二次方成正比;金在碘化物溶液中主要以AuI2-和AuI4-的形式存在。ANGELIDIS等[10]利用旋转圆盘技术对金在碘-碘化物体系中的溶解进行了研究,发现适当调整溶液中碘和碘化物的浓度,金的溶解即可自发进行,且金的溶解速率远比氰化物和硫脲的快。俄罗斯莫斯科国立贵金属勘探研究院研究证明:金-碘络合物的稳定性仅次于金-氰络合物的,优于其他金的络合物的稳定性[11]。针对含碳矿石(有机碳含量为1.6%)及氧化矿石[12]、戈塘金矿含碳原生矿[13]、浮选金精矿[14]、含铜难处理金矿[15]等进行的碘化浸出试验结果表明,碘化法具有良好的浸出效果。本文作者所在课题组[16]通过碘化物对金浸出效果的影响研究发现,碘化钾的浸出效果优于氢碘酸和碘化铵;并针对浮选金精矿进行了动力学研究,发现浸出反应过程主要受界面化学反应控制[17]。
MARUN等[18]和李桂春等[19]采用Pourbaix法分别绘制出了碘化浸金体系的φ-pH图。Pourbaix法是根据络合物逐级生成反应的平衡,确定简单离子与络合物离子各自的热力学稳定区。在有多种络合物存在的复杂浸金体系中,既存在简单离子又存在络合离子,并且简单离子与络合离子之间相互转化,存在动态平衡。采用Pourbaix法绘制有多种络合物存在的复杂浸金体系是不符合实际情况的。为了更为准确地绘制碘化浸金体系的φ-pH图和分析碘化浸金热力学变化规律,本文作者根据研究人员[20-22]提出的同时平衡原理,对Au-I--H2O系的浸金热力学进行了分析。主要研究了溶液与固体Au和AuI沉淀与固体Au平衡时的φ-pH关系、AuI存在条件和溶液中含金组分的变化规律。为金的高效碘化浸出提供了理论依据。
1 Au-I--H2O系的电化学平衡
1.1 溶液与固体Au的平衡
Au-I--H2O系是一个既含有简单金离子Au+和Au3+,又含有络合离子AuI2-和AuI4-的复杂体系,存在Au+、Au3+、AuI2-、AuI4-与固体Au的电化学反应,分别如式(1)~(4)所示:
1) Au+与固体Au的平衡
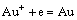 ,
,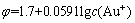 (1)
(1)
2) Au3+与固体Au的平衡
 ,
, (2)
(2)
3) AuI2-与固体Au的平衡
 ,
,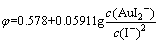 (3)
(3)
4) AuI4-与固体Au的平衡
 ,
, (4)
(4)
因此,采用同时平衡原理计算Au-I--H2O系的φsol/Au,必须同时考虑溶液中这4种离子与固体金的平衡。
Au-I--H2O系中无论哪种离子与固体金形成平衡,其还原电位都是相等的,即Au-I--H2O系中还原电位只有1个,式(1)~(4)中的φ相等。设cT(Au)为溶液中含金组分的总浓度,cT(I-)为溶液中含碘组分的总浓度,则有溶液平衡方程如式(5)和(6)所示:
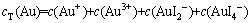 (5)
(5)
 (6)
(6)
将式(1)~(4)分别代入式(5)和(6)中,可得:
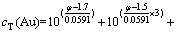
 (7)
(7)

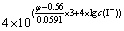 (8)
(8)
式中:φ为还原电位,V;c(I-)为溶液中游离的I-离子浓度,mol/L。
根据式(7)和(8)可知,在温度恒定的情况下,影响溶液与固体Au平衡的变量有4个,即cT(Au)、cT(I-)、φ和c(I-)。在这4个变量之间有式(7)和(8)两个方程,当固定cT(Au)和cT(I-)时,独立变量就变成了2个。因此,通过固定cT(Au)及改变cT(I-),可以求得与各cT(I-)相对应的φ和c(I-)。根据不同含金离子与固体金的平衡,还可以确定Au+、Au3+、AuI2-和AuI4-的平衡浓度。
1.2 AuI沉淀与固体Au的平衡
在一定条件下Au-I--H2O系中还会出现AuI沉淀,即除上述溶液与固体Au的平衡外,还有AuI沉淀与固体Au的平衡如式(9)所示:
 ,
, (9)
(9)
为了确定溶液中AuI沉淀存在的条件,还需要计算φAuI/Au。根据同时平衡原理,将式(1)~(4)和(9)分别代入式(5)和式(6),可得:
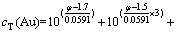
 (10)
(10)
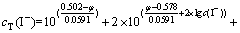
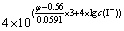 (11)
(11)
根据式(10)和(11)可知,在温度恒定的情况下,影响存在AuI沉淀的Au-I--H2O系平衡的变量也有4个,即cT(Au)、cT(I-)、φ和c(I-)。在这4个变量之间有式(10)和(11)两个方程,当固定cT(Au)和cT(I-)时,独立变量就变成了2个。因此,通过固定cT(Au)及改变cT(I-),可求出与各cT(I-)相对应的φ和c(I-)。同样根据不同含金离子与固体金的平衡,确定相应含金离子的平衡浓度。
2 Au-I--H2O系热力学分析
一般在Au-H2O系中,除了金的氧化反应,还存在的化学反应如式(12)所示:

 (12)
(12)
在高pH值条件下Au还会与OH-反应生成不同的含金阴离子,如H2AuO3-、HAuO32-和AuO33-。由于碘化浸金过程中溶液的pH值一般控制在中性左右[23],为了更加简洁明了地在φ-pH图中突显I-离子浓度对φ和pH值的影响,实验中忽略H2AuO3-、HAuO32-和AuO33-等阴离子在φ-pH图中的存在区域。
2.1 Au-I--H2O系φ-pH图
根据溶液与固体Au的同时平衡方程和固体AuI与固体Au的同时平衡方程及式(12),绘制的不同溶液cT(Au)和cT(I-)的Au-I--H2O系φ-pH图如图1和2所示。
图1所示为25 ℃下溶液中cT(I-)为10 mol/L、cT(Au)分别为1和1×10-4 mol/L时的φ-pH图。由图1可以看出,Au-I--H2O系溶液中cT(Au)为1 mol/L、cT(I-)为10 mol/L时,φsol/Au由金的标准还原电位1.5 V降低到0.47 V,φAuI/Au为0.449 V,φsol/Au高于φAuI/Au,此时溶液中会出现AuI沉淀。当溶液中的cT(Au)降低到10-4 mol/L时,φsol/Au进一步降至0.223 V,低于φAuI/Au的0.443 V,在溶液的稳定区域内不会出现AuI沉淀,而会发生AuI沉淀进一步溶解的反应如式(13)所示:
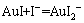 (13)
(13)
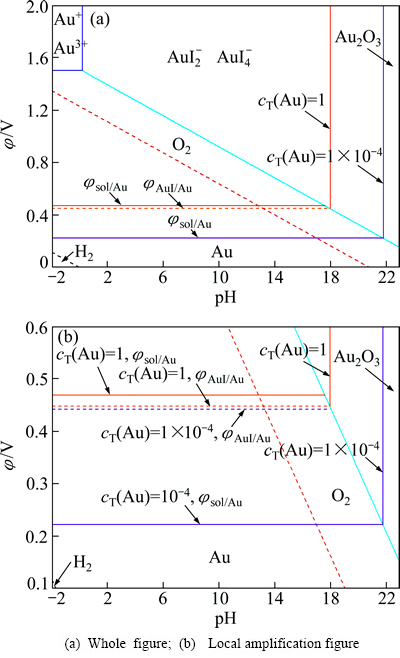
图1 Au-I--H2O系φ-pH图
Fig. 1 φ-pH diagrams for Au-I--H2O system (cT(I-)=10 mol/L, cT(Au)=1 mol/L, 10-4 mol/L)
因此,在Au-I--H2O系中降低溶液中cT(Au)可降低φsol/Au和避免AuI沉淀的生成,有利于促进金的碘化浸出。
图2所示为25 ℃下溶液中cT(Au)=10-4 mol/L,cT(I-)分别为1×10-4、1和10 mol/L时的φ-pH图。由图2可以看出,Au-I--H2O系溶液中cT(Au)为1×10-4 mol/L时,随着cT(I-)的增加,φsol/Au与φAuI/Au都逐步降低,Au3+/Au2O3的平衡pH值增大。当溶液中cT(I-)为1×10-4 mol/L时,φsol/Au高于φAuI/Au,这种情况下溶液中会出现AuI沉淀;当溶液中的cT(I-)增加到1 mol/L时, φsol/Au为0.342 V,低于φAuI/Au的0.502 V,溶液的稳定区域内不会出现AuI沉淀,此时Au3+/Au2O3的平衡pH值增大到19.8;继续增加溶液中的cT(I-)到10 mol/L时,φsol/Au为0.223 V,远低于φAuI/Au的0.443 V,溶液同样不会有AuI沉淀,Au3+/Au2O3的平衡pH值增大到21.8。因此,在Au-I--H2O系中增加溶液中cT(I-)浓度可降低φsol/Au和避免溶液中出现AuI沉淀,对金的碘化浸出非常有利。

图2 Au-I--H2O系φ-pH图
Fig. 2 φ-pH diagram for Au-I--H2O system (cT(Au)=1×10-4 mol/L, cT(I-)=1×10-4, 1, 10 mol/L)
2.2 Au-I--H2O系φ-lgcT(I-)图
在溶液cT(Au)为1×10-4 mol/L的Au-I--H2O系中,为了分析AuI沉淀出现的条件和区域,根据同时平衡原理可以计算出不同cT(I-)时溶液的还原电位φ,还原电位φ与lgcT(I-)的关系如图3所示。
由图3可以看出,随着溶液中cT(I-)的增加,电位逐渐降低。当cT(I-)增加到0.0021 mol/L时,电位降低到0.663 V,继续增加cT(I-)至0.1 mol/L时,电位降低到0.46 V。图3中两条线包围的部分为AuI出现的区域。随着cT(I-)的增加,上下两条线的电位差先增大后减小,说明溶液中AuI沉淀量呈先增加后减小的趋势。当cT(I-)增加到0.0021 mol/L时,AuI沉淀逐渐溶解、消失,两条线交于一点,该点为AuI沉淀存在与否的转折点。继续增大cT(I-),AuI沉淀彻底消失。因此,碘化浸金过程中加入适量的I-离子可以促进金的浸出,并避免AuI沉淀的出现。

图3 Au-I--H2O系φ-lgcT(I-)图
Fig. 3 φ-lgcT(I-) diagram for Au-I--H2O system (cT(Au)=10-4 mol/L)
2.3 Au-I--H2O系含金组分分布图
在已知溶液cT(Au)的Au-I--H2O系中,根据同时平衡原理可以计算出不同溶液cT(I-)时各种含金组分的浓度和分布率。图4所示为溶液中cT(Au)为1×10-4 mol/L时Au-I--H2O系中含金组分分布规律。
由图4可知,溶液中cT(I-)对Au-I--H2O系的含金组分分布影响很大。当溶液中cT(I-)小于10-6 mol/L时,金主要以Au+和Au3+的形式存在,由于cT(I-)太小,可以将Au-I--H2O系近似看作Au-H2O系;随着溶液中cT(I-)的增加,出现AuI沉淀且其沉淀量呈先增加后减小的趋势,溶液中c(Au+)、c(Au3+)、c(AuI2-)减少,c(AuI4-)增加;当cT(I-)增加到0.0021 mol/L时,AuI沉淀逐渐溶解、消失(见式(13)),继续增加溶液中cT(I-),c(AuI4-)浓度减少并趋于0,c(AuI2-)浓度增加,成为溶液中金的主要存在形式。

图4 cT(I-)对Au-I--H2O系中含金组分分布率的影响
Fig. 4 Effect of cT(I-) concentration in Au-I--H2O system on distribution rate of gold-bearing components (cT(Au)=1×10-4 mol/L)
3 碘化浸金实验研究
根据上述碘化浸金热力学分析结果,以某浮选金精矿和某含铜难处理氧化金矿为研究对象分别进行碘化浸出实验。在温度为25 ℃、液固比为4:1、pH值为7.5、搅拌速度为400 r/min、碘初始浓度为8 g/L、金精矿浸出4 h、含铜氧化金矿浸出2 h的条件下,分别进行碘与I-离子质量比为1:2、1:3、1:4、1:5、1:6和1:8的浸出试验,实验结果如图5所示。由图5可知,在初始碘浓度一定的条件下,随着I-离子浓度的增加,金的浸出率逐渐升高,当碘与I-离子质量比为1:6时,金浸出率达到88%左右。此试验结果与上述热力学分析中增加I-离子浓度对促进金的浸出结果相一致。

图5 碘与I-离子质量比对金浸出率的影响
Fig. 5 Effect of mass ratio of iodine and iodine ion on leaching rate of gold
4 结论
1) 采用同时平衡原理分析了Au-I--H2O系的浸金热力学,研究了溶液与固体Au和固体AuI与固体Au平衡时的φ-pH关系,随着溶液中cT(Au)的降低或cT(I-)的增加,φsol/Au迅速降低,Au3+/Au2O3的平衡pH值增大,非常有利于金的碘化浸出。
2) 在溶液cT(Au)为1×10-4 mol/L的Au-I--H2O系中,随着溶液中cT(I-)的逐渐增加,出现AuI沉淀且其沉淀量呈先增加后减小的趋势。当cT(I-)增加到0.0021 mol/L时,AuI沉淀逐渐溶解、消失。
3) 在溶液cT(Au)为1×10-4 mol/L的Au-I--H2O系中,随着溶液中cT(I-)的逐渐增加,c(Au+)和c(Au3+)减小,c(AuI4-)先增加后减小,c(AuI2-)逐渐增加。当cT(I-)大于0.0021 mol/L时,溶液中的金主要以AuI2-络合离子形式存在。
4) 碘化浸金实验结果表明,采用碘化法浸出浮选金精矿和含铜难处理氧化金矿均获得了较好的效果,金的浸出率达到88%左右。
REFERENCES
[1] 吕进云. 碘化浸金机理及工艺条件的研究[D]. 哈尔滨: 黑龙江科技学院, 2010: 2-3.
L Jin-yun. Study on the mechanism and technical conditions of iodine leaching of gold[D]. Haerbing: Heilongjiang Institute of Science and Technology, 2010: 2-3.
Jin-yun. Study on the mechanism and technical conditions of iodine leaching of gold[D]. Haerbing: Heilongjiang Institute of Science and Technology, 2010: 2-3.
[2] WILMOT J C, MILOSAVLJEVIC E B, SOLUJIC L, HENDRIX J L. Fate of cyanide in gold ores containing reduced sulphur minerals[C]//Proceedings of 24th International Mineral Processing Congress. Beijing: Science Press, 2008: 2958-2964.
[3] DAI X, JEFFREY M I. The effect of sulfide minerals on the leaching of gold in aerated cyanide solutions[J]. Hydrometallurgy, 2006, 82(3/4): 118-125.
[4] 玉 涵, 胡显智. 氰化及非氰化提金方法综述[J]. 云南冶金, 2010, 39(3): 9-12.
YU Han, HU Xian-zhi. Review of gold-leaching technologies by cyanidation and non-cyanidation[J]. Yunnan Metallurgy, 2010, 39(3): 9-12.
[5] 李桂春, 卢寿慈. 非氰化提金技术的发展[J]. 中国矿业, 2003, 12(3): 1-5.
LI Gui-chun, LU Shou-ci. The development of non-cyanidation gold lixiviating technology[J]. China Mining Magazine, 2003, 12(3): 1-5.
[6] HILSON G, MONHEMIUS A J. Alternatives to cyanide in the gold mining industry: what prospects for the future[J]. Journal of Cleaner Production, 2006, 14(12/13): 1158-1167.
[7] SYED S. Recovery of gold from secondary sources—A review[J]. Hydrometallurgy, 2012, 115/116: 30-51.
[8] QI P H, HISKEY J B. Dissolution kinetics of gold in iodide solutions[J]. Hydrometallurgy, 1991, 27(1): 47-62.
[9] QI P H, HISKEY J B. Electrochemical behavior of gold in iodide solutions[J]. Hydrometallurgy, 1993, 32(2): 161-179.
[10] ANGELIDIS T N, KYDROS K A, MATIS K A. A fundamental rotating disk study of gold dissolution in iodine-iodide solutions[J]. Hydrometallurgy, 1993, 34(1): 49-64.
[11] SEDELNIKOVA G V, KRYLOV G S. Iodinated and brominated solvent of gold[J]. Schnigri, 2001, 3: 43-52.
[12] BAGHALHA M. The leaching kinetics of an oxide gold ore with iodide/iodine solutions[J]. Hydrometallurgy, 2012, 113/114: 42-50.
[13] 李桂春, 卢寿慈. 碘化浸金试验研究[J]. 中国矿业, 2004, 13(7): 66-68.
LI Gui-chun, LU Shou-ci. Experimental investigation for iodine leaching of gold[J]. China Mining Magazine, 2004, 13(7): 66-68.
[14] WANG Hai-xia, SUN Chun-bao, LI Shao-ying. Study on gold concentrate leaching by iodine-iodide[J]. International Journal of Minerals, Metallurgy and Materials, 2013, 20(4): 323-328.
[15] 袁喜振,李绍英,赵留成,康金星,孙春宝,李根壮,张 炜.某含铜难处理金矿选择性浸出试验研究[J].中国科技论文在线, 2014, 9(3): 351-354.
YUAN Xi-zhen, LI Shao-ying, ZHAO Liu-cheng, KANG Jin-xing, SUN Chun-bao, LI Gen-zhuang, ZHANG Wei. Experimental study on selective leaching of refractory gold ores[J]. China Sciencepaper Online, 2014, 9(3): 351-354.
[16] 李绍英, 王海霞, 孙春宝, 赵留成, 阎志强. 碘化物对金精矿碘化浸出的影响[J]. 中国有色金属学报, 2013, 23(5): 1434-1439.
LI Shao-ying, WANG Hai-xia, SUN Chun-bao, ZHAO Liu-cheng, YAN Zhi-qiang. Effect of different iodide on gold concentrates leaching process in iodine-iodide solution[J]. The Chinese Journal of Nonferrous Metals, 2013, 23(5): 1434-1439.
[17] 李绍英, 王海霞, 袁喜振, 赵留成, 孙春宝. 金精矿碘化浸出过程动力学[J]. 中国有色金属学报, 2014, 24(3): 814-819.
LI Shao-ying, WANG Hai-xia, YUAN Xi-zhen, ZHAO Liu-cheng, SUN Chun-bao. Leaching dynamics of gold concentrates by iodine-iodide solution[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(3): 814-819.
[18] MARUN J N, MEISSL R J, LARA R F, GARCIA R A. Gold bearing ore processing with iodine-iodide solutions[C]// Proceedings of the XX International Mineral Processing Congress, 1997: 381-389.
[19] 李桂春, 吕进云. 碘化浸金过程的基本理论[J]. 黑龙江科技学院学报, 2009, 19(5): 345-347, 364.
LI Gui-chun, L Jin-yun. Basic theory of iodide leaching of gold process[J]. Journal of Heilongjiang Institute of Science and Technology, 2009, 19(5): 345-347, 364.
Jin-yun. Basic theory of iodide leaching of gold process[J]. Journal of Heilongjiang Institute of Science and Technology, 2009, 19(5): 345-347, 364.
[20] 钟竹前, 梅光贵, 蔡传算. Au-Cl--H2O系的热力学分析[J]. 黄金, 1982(3): 44-49.
ZHONG Zhu-qian, MEI Guang-gui, CAI Chuan-suan. The thermodynamic analysis for Au-Cl--H2O system[J]. Gold, 1982(3): 44-49.
[21] 蒙星辉. Au-NH3-H2O系的电位-pH图及其热力学分析[J]. 化工冶金, 1988, 9(3): 56-60.
MENG Xing-hui. E-pH diagrams and thermodynamic analysis of Au-NH3-H2O system[J]. Engineering Chemistry and Metallurgy, 1988, 9 (3): 56-60.
[22] 傅崇说, 郑蒂基. 关于Cu-Cl--H2O系的热力学分析及电位-pH图[J]. 中南矿冶学院学报, 1980(3): 12-24.
FU Chong-yue, ZHENG Di-ji. The thermodynamic analysis on the Cu-Cl--H2O system and its potential-pH diagram[J]. Journal of Central South Institute of Mining and Metallurgy, 1980(3): 12-24.
[23] DAVIS A, TRAN T, YOUNG D R. Solution chemistry of iodide leaching of gold[J]. Hydrometallurgy, 1993, 32(2): 143-159.
(编辑 王 超)
基金项目:高等学校博士学科点专项科研基金资助项目(20100006110003)
收稿日期:2014-04-30;修订日期:2015-07-13
通信作者:孙春宝,教授,博士;电话:010-62334953;E-mail:suncb@ustb.edu.cn